Kangtaizhi Granule Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats and HepG2 Cells via AMPK/mTOR Signaling Pathway
- PMID: 32884949
- PMCID: PMC7455821
- DOI: 10.1155/2020/3413186
Kangtaizhi Granule Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats and HepG2 Cells via AMPK/mTOR Signaling Pathway
Abstract
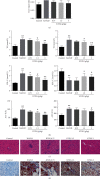
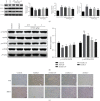
Kangtaizhi granule (KTZG) is a Chinese medicine compound prescription and has been proven to be effective in nonalcoholic fatty liver disease (NAFLD) treatment clinically. However, the underlying mechanisms under this efficacy are rather elusive. In the present study, network pharmacology and HPLC analysis were performed to identify the chemicals of KTZG and related target pathways for NAFLD treatment. Network pharmacology screened 42 compounds and 79 related targets related to NAFLD; HPLC analysis also confirmed six compounds in KTZG. Further experiments were also performed. In an in vivo study, SD rats were randomly divided into five groups: control (rats fed with normal diet), NAFLD (rats fed with high-fat diet), and KTZG 0.75, 1.5, and 3 groups (NAFLD rats treated with KTZG 0.75, 1.5, and 3 g/kg, respectively). Serum lipids were biochemically determined; hepatic steatosis and lipid accumulation were evaluated with HE and oil red O staining. In an in vitro study, HepG2 cells were incubated with 1 mM FFA to induce lipid accumulation with or without KTZG treatment. MTT assay, intracellular TG level, oil red O staining, and glucose uptake in cells were detected. Western blotting and immunohistochemical and immunofluorescence staining were also performed to determine the expression of lipid-related genes PPAR-γ, SREBP-1, p-AKT, FAS, and SIRT1 and genes in the AMPK/mTOR signaling pathway. In high-fat diet-fed rats, KTZG treatment significantly improved liver organ index and serum lipid contents of TG, TC, LDL-C, HDL-C, ALT, and AST significantly; HE and oil red O staining also showed that KTZG alleviated hepatic steatosis and liver lipid accumulation. In FFA-treated HepG2 cells, KTZG treatment decreased the intracellular TG levels, lipid accumulation, and attenuated glucose uptake significantly. More importantly, lipid-related genes PPAR-γ, SREBP-1, p-AKT, FAS, and SIRT1 expressions were ameliorated with KTZG treatment in high-fat diet-fed rats and FFA-induced HepG2 cells. The p-AMPK and p-mTOR expressions in the AMPK/mTOR signaling pathway were also modified with KTZG treatment in high-fat diet-fed rats and HepG2 cells. These results indicated that KTZG effectively ameliorated lipid accumulation and hepatic steatosis to prevent NAFLD in high-fat diet-fed rats and FFA-induced HepG2 cells, and this effect was associated with the AMPK/mTOR signaling pathway. Our results suggested that KTZG might be a potential therapeutic agent for the prevention of NAFLD.
Copyright © 2020 Jiaxin Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures







References
-
- Dang Y. Q., Hao S. J., Zhou W. J., Zhang L., Ji G. The traditional Chinese formulae Ling-gui-zhu-gan decoction alleviated non-alcoholic fatty liver disease via inhibiting PPP1R3C mediated molecules. BMC Complementary and Alternative Medicine. 2019;19(1):p. 8. doi: 10.1186/s12906-018-2424-1. - DOI - PMC - PubMed
-
- Cotter T. G., Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;157(7):1851–1864. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

