Computed tomography guided sizing for transcatheter pulmonary valve replacement
- PMID: 32885027
- PMCID: PMC7452563
- DOI: 10.1016/j.ijcha.2020.100523
Computed tomography guided sizing for transcatheter pulmonary valve replacement
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Int J Cardiol Heart Vasc. 2020 Nov 18;31:100676. doi: 10.1016/j.ijcha.2020.100676. eCollection 2020 Dec. Int J Cardiol Heart Vasc. 2020. PMID: 33364333 Free PMC article.
Abstract
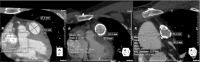
Objective: To evaluate the predictive value of Computed Tomography Angiography (CTA) measurements of the RVOT for transcatheter valve sizing.
Background: Transcatheter pulmonary valve replacement (TPVR) provides an alternative to surgery in patients with right ventricular outflow tract (RVOT) dysfunction. We studied 18 patients who underwent catheterization for potential TPVR to determine whether CT imaging can be used to accurately predict implant size.
Methods: Cases were grouped by RVOT characteristics: native or transannular patch (n = 8), conduit (n = 5) or bioprosthetic valve (n = 5). TPVR was undertaken in 14/18 cases, after balloon-sizing was used to confirm suitability and select implant size. Retrospective CT measurements of the RVOT (circumference-derived (Dcirc) and area-derived (Darea) diameters) were obtained at the level of the annulus, bioprosthesis or conduit. Using manufacturer sizing guidance, a valve size was generated and a predicted valve category assigned: (1) <18 mm, (2) 18-20 mm, (3) 22-23 mm, (4) 26-29 mm and (5) >29 mm. Predicted and implanted valves were compared for inter-rater agreement using Cohen's kappa coefficient.
Results: The median age of patients was 37 years old (IQR: 30-49); 55% were male. Diagnoses included: Tetralogy of Fallot (12/18), d-Transposition repair (3/18), congenital pulmonary stenosis (2/18) and carcinoid heart disease (1/18). Measurements of Darea (κ = 0.697, p < 0.01) and Dcirc (κ = 0.540, p < 0.01) were good predictors of implanted valve size. When patients with RVOT conduits were excluded, the predictive accuracy improved for Darea (κ = 0.882, p < 0.01) and Dcirc (κ = 0.882, p < 0.01).
Conclusions: CT measurement of the RVOT, using Darea or Dcirc, can predict prosthetic valve sizing in TPVR. These measurements are less predictive in patients with conduits, compared to those with a native RVOT or pulmonic bioprosthesis.
Condensed abstract: We studied 18 patients who underwent catheterization for TPVR to determine whether CT imaging could be used to accurately predict implant size. Retrospective RVOT measurements were used to generate a predicted valve size, which was compared with implanted valve size for inter-rater agreement. Measurements of Darea (κ = 0.697, p < 0.01) and Dcirc (κ = 0.540, p < 0.01) were good predictors of implanted valve size. When cases with RVOT conduits were excluded, the predictive accuracy improved for Darea (κ = 0.882, p < 0.01) and Dcirc (κ = 0.882, p < 0.01). CT measurement of the RVOT can accurately predict prosthetic valve sizing in TPVR. These measurements are less predictive in patients with conduits.
Keywords: Darea, Area-derived Diameter; Dcirc, Circumference-derived Diameter; RVOT, Right Ventricular Outflow; TAVR, Transcatheter Aortic Valve Replacement; TPVR, Transcatheter Pulmonary Valve Replacement.
© 2020 Published by Elsevier B.V.
Figures


References
-
- Bonhoeffer P., Boudjemline Y., Saliba Z. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. - PubMed
-
- Coats L., Khambadkone S., Derrick G. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113:2037–2044. - PubMed
-
- Lurz P., Nordmeyer J., Giardini A. Early versus late functional outcome after successful percutaneous pulmonary valve implantation: are the acute effects of altered right ventricular loading all we can expect? J. Am. Coll. Cardiol. 2011;57:724–731. - PubMed
-
- Lurz P., Coats L., Khambadkone S. Percutaneous pulmonary valve implantation: Impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

