Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomised clinical trial
- PMID: 32885276
- PMCID: PMC8273663
- DOI: 10.1007/s00134-020-06216-x
Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomised clinical trial
Abstract
Purpose: ICU discharge is often delayed by a requirement for intravenous vasopressor medications to maintain normotension. We hypothesised that the administration of midodrine, an oral α1-adrenergic agonist, as adjunct to standard treatment shortens the duration of intravenous vasopressor requirement.
Methods: In this multicentre, randomised, controlled trial including three tertiary referral hospitals in the US and Australia, we enrolled adult patients with hypotension requiring a single-agent intravenous vasopressor for ≥ 24 h. Subjects received oral midodrine (20 mg) or placebo every 8 h in addition to standard care until cessation of intravenous vasopressors, ICU discharge, or occurrence of adverse events. The primary outcome was time to vasopressor discontinuation. Secondary outcomes included time to ICU discharge readiness, ICU and hospital lengths of stay, and ICU readmission rates.
Results: Between October 2012 and June 2019, 136 participants were randomised, of whom 132 received the allocated intervention and were included in the analysis (modified intention-to-treat approach). Time to vasopressor discontinuation was not different between midodrine and placebo groups (median [IQR], 23.5 [10-54] vs 22.5 [10.4-40] h; difference, 1 h; 95% CI - 10.4 to 12.3 h; p = 0.62). No differences in secondary endpoints were observed. Bradycardia occurred more often after midodrine administration (5 [7.6%] vs 0 [0%], p = 0.02).
Conclusion: Midodrine did not accelerate liberation from intravenous vasopressors and was not effective for the treatment of hypotension in critically ill patients.
Keywords: ICU discharge; Midodrine; Oral vasopressor; Persistent hypotension.
Conflict of interest statement
Figures
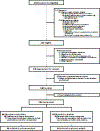

Comment in
-
Midodrine administration during critical illness: fixed-dose or titrate to response?Intensive Care Med. 2021 Feb;47(2):249-251. doi: 10.1007/s00134-020-06321-x. Epub 2020 Nov 25. Intensive Care Med. 2021. PMID: 33237345 Free PMC article. No abstract available.
-
High-dose midodrine is not effective for treatment of persistent hypotension in the intensive care unit.Intensive Care Med. 2021 Feb;47(2):252-253. doi: 10.1007/s00134-020-06333-7. Epub 2021 Jan 8. Intensive Care Med. 2021. PMID: 33416917 No abstract available.
-
Options to Minimize the Use of Central Lines: Midodrine, Peripheral Venous Lines, and Midline Catheters.Am J Respir Crit Care Med. 2021 Dec 15;204(12):1473-1475. doi: 10.1164/rccm.202103-0685RR. Am J Respir Crit Care Med. 2021. PMID: 34699334 No abstract available.
References
-
- Thongprayoon C, Cheungpasitporn W, Harrison AM, Carrera P, Srivali N, Kittamongkolchai W, Erdogan A, Kashani KB (2016) Temporal trends in the utilization of vasopressors in intensive care units: an epidemiologic study. BMC Pharmacol Toxicol 17 (1):19. doi:10.1186/s40360-016-0063-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

