Does "Birth" as an Event Impact Maturation Trajectory of Renal Clearance via Glomerular Filtration? Reexamining Data in Preterm and Full-Term Neonates by Avoiding the Creatinine Bias
- PMID: 32885464
- PMCID: PMC7818478
- DOI: 10.1002/jcph.1725
Does "Birth" as an Event Impact Maturation Trajectory of Renal Clearance via Glomerular Filtration? Reexamining Data in Preterm and Full-Term Neonates by Avoiding the Creatinine Bias
Abstract
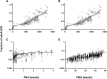
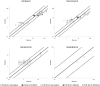
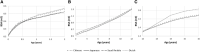
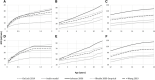
Glomerular filtration rate (GFR) is an important measure of renal function. Various models for its maturation have recently been compared; however, these have used markers, which are subject to different renal elimination processes. Inulin clearance data (a purer probe of GFR) collected from the literature were used to determine age-related changes in GFR aspects of renal drug excretion in pediatrics. An ontogeny model was derived using a best-fit model with various combinations of covariates such as postnatal age, gestational age at birth, and body weight. The model was applied to the prediction of systemic clearance of amikacin, gentamicin, vancomycin, and gadobutrol. During neonatal life, GFR increased as a function of both gestational age at birth and postnatal age, hence implying an impact of birth and a discrepancy in GFR for neonates with the same postmenstrual age depending on gestational age at birth (ie, neonates who were outside the womb longer had higher GFR, on average). The difference in GFR between pre-term and full-term neonates with the same postmenstrual age was negligible from beyond 1.25 years. Considering both postnatal age and gestational age at birth in GFR ontogeny models is important because postmenstrual age alone ignores the impact of birth. Most GFR models use covariates of body size in addition to age. Therefore, prediction from these models will also depend on the change in anthropometric characteristics with age. The latter may not be similar in various ethnic groups, and this makes the head-to-head comparison of models very challenging.
Keywords: birth; glomerular filtration rate; maturation; neonates.
© 2020 The Authors. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
F.S. and T.N.J. are employed full‐time and A.B.J.H. and A.R.H. part‐time by Certara, a company that develops and supplies modeling and simulation software and services to the pharmaceutical industry. A.R.H. is also director of the Centre for Applied Pharmacokinetic Research at the University of Manchester.
Figures


References
-
- Allegaert K, Scheers I, Cossey V, Anderson BJ. Covariates of amikacin clearance in neonates: the impact of postnatal age on predictability. Drug Metab Lett. 2008;2(4):286‐289. - PubMed
-
- Sherwin CM, Svahn S, Van der Linden A, Broadbent RS, Medlicott NJ, Reith DM. Individualised dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2009;65(7):705‐713. - PubMed
-
- Botha JH, du Preez MJ, Adhikari M. Population pharmacokinetics of gentamicin in South African newborns. Eur J Clin Pharmacol. 2003;59(10):755‐759. - PubMed
-
- DiCenzo R, Forrest A, Slish JC, Cole C, Guillet R. A gentamicin pharmacokinetic population model and once‐daily dosing algorithm for neonates. Pharmacotherapy. 2003;23(5):585‐591. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

