Cyanine-Flavonol Hybrids for Near-Infrared Light-Activated Delivery of Carbon Monoxide
- PMID: 32885885
- PMCID: PMC7693251
- DOI: 10.1002/chem.202003272
Cyanine-Flavonol Hybrids for Near-Infrared Light-Activated Delivery of Carbon Monoxide
Abstract
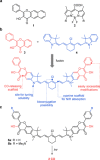
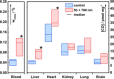
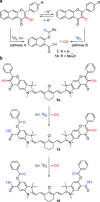
Carbon monoxide (CO) is an endogenous signaling molecule that controls a number of physiological processes. To circumvent the inherent toxicity of CO, light-activated CO-releasing molecules (photoCORMs) have emerged as an alternative for its administration. However, their wider application requires photoactivation using biologically benign visible and near-infrared (NIR) light. In this work, a strategy to access such photoCORMs by fusing two CO-releasing flavonol moieties with a NIR-absorbing cyanine dye is presented. These hybrids liberate two molecules of CO in high chemical yields upon activation with NIR light up to 820 nm and exhibit excellent uncaging cross-sections, which surpass the state-of-the-art by two orders of magnitude. Furthermore, the biocompatibility and applicability of the system in vitro and in vivo are demonstrated, and a mechanism of CO release is proposed. It is hoped that this strategy will stimulate the discovery of new classes of photoCORMs and accelerate the translation of CO-based phototherapy into practice.
Keywords: CO release; cyanine; near-infrared light; photoCORM; photorelease.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
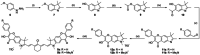

References
-
- Phan T. G., Bullen A., Immunol. Cell Biol. 2010, 88, 438–444. - PubMed
-
- Lim Y. T., Kim S., Nakayama A., Stott N. E., Bawendi M. G., Frangioni J. V., Mol. Imaging 2003, 2, 50–64. - PubMed
-
- Allison R. R., Sibata C. H., Photodiagn. Photodyn. Ther. 2010, 7, 61–75. - PubMed
-
- Romão C. C., Blättler W. A., Seixas J. D., Bernardes G. J. L., Chem. Soc. Rev. 2012, 41, 3571–3583. - PubMed
-
- Motterlini R., Otterbein L. E., Nat. Rev. Drug Discovery 2010, 9, 728–743. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

