Physiologically based pharmacokinetic modeling to assess metabolic drug-drug interaction risks and inform the drug label for fedratinib
- PMID: 32886148
- PMCID: PMC7515950
- DOI: 10.1007/s00280-020-04131-y
Physiologically based pharmacokinetic modeling to assess metabolic drug-drug interaction risks and inform the drug label for fedratinib
Abstract
Purpose: Fedratinib (INREBIC®), a Janus kinase 2 inhibitor, is approved in the United States to treat patients with myelofibrosis. Fedratinib is not only a substrate of cytochrome P450 (CYP) enzymes, but also exhibits complex auto-inhibition, time-dependent inhibition, or mixed inhibition/induction of CYP enzymes including CYP3A. Therefore, a mechanistic modeling approach was used to characterize pharmacokinetic (PK) properties and assess drug-drug interaction (DDI) potentials for fedratinib under clinical scenarios.
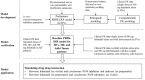
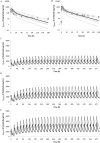
Methods: The physiologically based pharmacokinetic (PBPK) model of fedratinib was constructed in Simcyp® (V17R1) by integrating available in vitro and in vivo information and was further parameterized and validated by using clinical PK data.
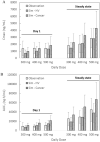
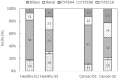
Results: The validated PBPK model was applied to predict DDIs between fedratinib and CYP modulators or substrates. The model simulations indicated that the fedratinib-as-victim DDI extent in terms of geometric mean area under curve (AUC) at steady state is about twofold or 1.2-fold when strong or moderate CYP3A4 inhibitors, respectively, are co-administered with repeated doses of fedratinib. In addition, the PBPK model successfully captured the perpetrator DDI effect of fedratinib on a sensitive CY3A4 substrate midazolam and predicted minor effects of fedratinib on CYP2C8/9 substrates.
Conclusions: The PBPK-DDI model of fedratinib facilitated drug development by identifying DDI potential, optimizing clinical study designs, supporting waivers for clinical studies, and informing drug label claims. Fedratinib dose should be reduced to 200 mg QD when a strong CYP3A4 inhibitor is co-administered and then re-escalated to 400 mg in a stepwise manner as tolerated after the strong CYP3A4 inhibitor is discontinued.
Keywords: Drug–drug interaction; Fedratinib; Metabolic inhibition; PBPK; Simcyp.
Conflict of interest statement
F.W., G.K, and S.S. are employees and hold equity ownership in Bristol Myers Squibb.
Figures

Similar articles
-
Advancements in physiologically based pharmacokinetic modeling for fedratinib: updating dose guidance in the presence of a dual inhibitor of CYP3A4 and CYP2C19.Cancer Chemother Pharmacol. 2024 Oct;94(4):549-559. doi: 10.1007/s00280-024-04696-y. Epub 2024 Aug 7. Cancer Chemother Pharmacol. 2024. PMID: 39110202
-
Drug-drug interaction (DDI) assessments of ruxolitinib, a dual substrate of CYP3A4 and CYP2C9, using a verified physiologically based pharmacokinetic (PBPK) model to support regulatory submissions.Drug Metab Pers Ther. 2019 May 30;34(2):/j/dmdi.2019.34.issue-2/dmpt-2018-0042/dmpt-2018-0042.xml. doi: 10.1515/dmpt-2018-0042. Drug Metab Pers Ther. 2019. PMID: 31145690
-
Assessment of effects of repeated oral doses of fedratinib on inhibition of cytochrome P450 activities in patients with solid tumors using a cocktail approach.Cancer Chemother Pharmacol. 2020 Jul;86(1):87-95. doi: 10.1007/s00280-020-04102-3. Epub 2020 Jun 14. Cancer Chemother Pharmacol. 2020. PMID: 32537715 Clinical Trial.
-
Physiologically based pharmacokinetic model to predict drug-drug interactions with the antibody-drug conjugate enfortumab vedotin.J Pharmacokinet Pharmacodyn. 2024 Oct;51(5):417-428. doi: 10.1007/s10928-023-09877-5. Epub 2023 Aug 26. J Pharmacokinet Pharmacodyn. 2024. PMID: 37632598 Free PMC article. Review.
-
Value of Assessing 1-Hydroxymidazolam in Drug-Drug Interaction Studies with Midazolam as a Substrate of Cytochrome P450 3A.J Clin Pharmacol. 2024 Sep;64(9):1123-1129. doi: 10.1002/jcph.2447. Epub 2024 May 26. J Clin Pharmacol. 2024. PMID: 38797881 Review.
Cited by
-
Effects of strong and moderate CYP3A4 inducers on the pharmacokinetics of fedratinib in healthy adult participants.Cancer Chemother Pharmacol. 2021 Sep;88(3):369-377. doi: 10.1007/s00280-021-04292-4. Epub 2021 May 21. Cancer Chemother Pharmacol. 2021. PMID: 34019108 Clinical Trial.
-
Advancements in physiologically based pharmacokinetic modeling for fedratinib: updating dose guidance in the presence of a dual inhibitor of CYP3A4 and CYP2C19.Cancer Chemother Pharmacol. 2024 Oct;94(4):549-559. doi: 10.1007/s00280-024-04696-y. Epub 2024 Aug 7. Cancer Chemother Pharmacol. 2024. PMID: 39110202
-
Pharmacokinetic and Pharmacodynamic Drug-Drug Interactions: Research Methods and Applications.Metabolites. 2023 Jul 29;13(8):897. doi: 10.3390/metabo13080897. Metabolites. 2023. PMID: 37623842 Free PMC article. Review.
-
Application of physiologically based pharmacokinetics modeling in the research of small-molecule targeted anti-cancer drugs.Cancer Chemother Pharmacol. 2023 Oct;92(4):253-270. doi: 10.1007/s00280-023-04566-z. Epub 2023 Jul 19. Cancer Chemother Pharmacol. 2023. PMID: 37466731 Review.
-
Preclinical Systemic Pharmacokinetics, Dose Proportionality, and Central Nervous System Distribution of the ATM Inhibitor WSD0628, a Novel Radiosensitizer for the Treatment of Brain Tumors.J Pharmacol Exp Ther. 2024 Jul 18;390(2):260-275. doi: 10.1124/jpet.123.001971. J Pharmacol Exp Ther. 2024. PMID: 38858089 Free PMC article.
References
-
- FDA (2019) INREBIC® (fedratinib) capsules, for oral use (USA drug label)
-
- FDA (2019) NDA212327: Cross-Discipline Team Leader Review
-
- Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–1837. doi: 10.1124/dmd.115.065920. - DOI - PMC - PubMed
-
- Wagner C, Pan Y, Hsu V, Grillo JA, Zhang L, Reynolds KS, Sinha V, Zhao P. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet. 2015;54(1):117–127. doi: 10.1007/s40262-014-0188-4. - DOI - PubMed
-
- Wagner C, Pan Y, Hsu V, Sinha V, Zhao P. Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet. 2016;55(4):475–483. doi: 10.1007/s40262-015-0330-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

