Strategic Anti-SARS-CoV-2 Serology Testing in a Low Prevalence Setting: The COVID-19 Contact (CoCo) Study in Healthcare Professionals
- PMID: 32886335
- PMCID: PMC7472691
- DOI: 10.1007/s40121-020-00334-1
Strategic Anti-SARS-CoV-2 Serology Testing in a Low Prevalence Setting: The COVID-19 Contact (CoCo) Study in Healthcare Professionals
Abstract
Background: Serology testing is explored for epidemiological research and to inform individuals after suspected infection. During the coronavirus disease 2019 (COVID-19) pandemic, frontline healthcare professionals (HCP) may be at particular risk for infection. No longitudinal data on functional seroconversion in HCP in regions with low COVID-19 prevalence and low pre-test probability exist.
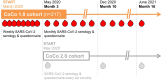
Methods: In a large German university hospital, we performed weekly questionnaire assessments and anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) measurements with various commercial tests, a novel surrogate virus neutralisation test, and a neutralisation assay using live SARS-CoV-2.
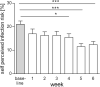
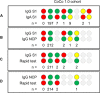
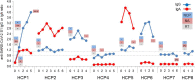
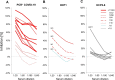
Results: From baseline to week 6, 1080 screening measurements for anti-SARS CoV-2 (S1) IgG from 217 frontline HCP (65% female) were performed. Overall, 75.6% of HCP reported at least one symptom of respiratory infection. Self-perceived infection probability declined over time (from mean 20.1% at baseline to 12.4% in week 6, p < 0.001). In sera of convalescent patients with PCR-confirmed COVID-19, we measured high anti-SARS-CoV-2 IgG levels, obtained highly concordant results from enzyme-linked immunosorbent assays (ELISA) using e.g. the spike 1 (S1) protein domain and the nucleocapsid protein (NCP) as targets, and confirmed antiviral neutralisation. However, in HCP the cumulative incidence for anti-SARS-CoV-2 (S1) IgG was 1.86% for positive and 0.93% for equivocal positive results over the study period of 6 weeks. Except for one HCP, none of the eight initial positive results were confirmed by alternative serology tests or showed in vitro neutralisation against live SARS-CoV-2. The only true seroconversion occurred without symptoms and mounted strong functional humoral immunity. Thus, the confirmed cumulative incidence for neutralizing anti-SARS-CoV-2 IgG was 0.47%.
Conclusion: When assessing anti-SARS-CoV-2 immune status in individuals with low pre-test probability, we suggest confirming positive results from single measurements by alternative serology tests or functional assays. Our data highlight the need for a methodical serology screening approach in regions with low SARS-CoV-2 infection rates.
Trial registration: The study is registered at DRKS00021152.
Keywords: COVID-19; Coronavirus; Healthcare professionals; Humoral immunity; Infection; Pandemic; SARS-CoV-2; Serological testing; Virus.
Figures
References
-
- Houlihan NVC, Byrne T, Lewer D, et al. SARS-CoV-2 virus and antibodies in front-line health care workers in an acute hospital in London: preliminary results from a longitudinal study. 2020. https://www.medrxiv.org/content/101101/2020060820120584v1article-info. Accessed 31 July 2020.
-
- Centers for Disease Control and Prevention Cluster of severe acute respiratory syndrome cases among protected health-care workers—Toronto, Canada, April 2003. MMWR Morb Mortal Wkly Rep. 2003;52(19):433–436. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

