Associations between safety, tolerability, and toxicity and the reporting of health-related quality of life in phase III randomized trials in common solid tumors
- PMID: 32886422
- PMCID: PMC7643655
- DOI: 10.1002/cam4.3390
Associations between safety, tolerability, and toxicity and the reporting of health-related quality of life in phase III randomized trials in common solid tumors
Abstract
Background: Anti-cancer drugs are approved typically on the basis of efficacy and safety as evaluated in phase III randomized trials (RCTs). Health-related quality of life (HRQoL) is a direct measure of patient benefit, but is under-reported. Here we explore associations with reporting of HRQoL data in phase III RCTs in common solid tumors.
Methods: We searched ClinicalTrials.gov to identify phase III RCTs evaluating new drugs in adults with advanced cancers that completed accrual between January 2005 and October 2016. Data on HRQoL, safety, and tolerability comprising treatment-related death, treatment discontinuation and commonly reported grade 3 or 4 adverse events (AEs) were extracted. Associations between these measures and reporting of HRQoL data were explored using logistic regression.
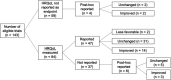
Results: Of 377 phase III RCTs identified initially, 143 studies were analysed and comprised 55% positive trials and 90% industry sponsored trials. HRQoL was listed as an endpoint in 59% trials; and of these, only 65% reported HRQoL data. There were higher odds of reporting HRQoL data for positive trials (OR 2.05, P = .04) and trials published in journals with higher impact factor (OR 1.35, P = .01). Reporting of HRQoL was not associated with treatment-related death (OR 1.25, P = .40) or treatment discontinuation (OR 1.12, P = .61), but was positively associated with dyspnea and dermatological adverse events.
Conclusions: HRQoL is reported in only two-thirds of RCTs that describe collecting such data. Reporting of HRQoL is associated with positive trial outcome and higher journal impact factor, but not associated with overall safety and tolerability of anti-cancer drugs.
Keywords: oncology; quality of life; randomized controlled trials; treatment toxicity.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr Eitan Amir reports personal fees from Genentech/Roche, personal fees from Apobiologix, personal fees from Myriad Genetics, personal fees from Agendia, outside the submitted work. Dr Ramy Saleh reports personal fees from Roche, outside the submitted work. Dr Nicholas Meti reports personal fees from Novartis, outside the submitted work. Dr Domen Ribnikar reports personal fees from Novartis, outside the submitted work. Dr Hadar Goldvaser reports personal honorarium fees from Roche, Novartis, Pfizer and Oncotest, personal advisory consultation fees from Novartis, all outside the submitted work. Dr Alberto Ocana reports travel support from Merck, advisory consultation fees from Daichii‐Sankyo and Entrechem. Dr Arnoud J. Templeton reports personal fees from Astellas, MSD, and Sanofi. Consultancy fees were paid to his institution by BMS, Janssen, Sanofi, and Roche. Conference/travel support was received from Sanofi, Janssen, Ipsen, and Roche. Dr Bostjan Seruga reports personal honorarium fees from Astellas, Jansse, Novartis and Roche.
Figures
Similar articles
-
Efficacy, safety and tolerability of drugs studied in phase 3 randomized controlled trials in solid tumors over the last decade.Sci Rep. 2021 May 25;11(1):10843. doi: 10.1038/s41598-021-90403-3. Sci Rep. 2021. PMID: 34035370 Free PMC article.
-
Analysis of Health-Related Quality of Life Reporting in Phase III RCTs of Advanced Genitourinary Tumors.Cancers (Basel). 2023 Dec 4;15(23):5703. doi: 10.3390/cancers15235703. Cancers (Basel). 2023. PMID: 38067406 Free PMC article.
-
The reporting of adverse events in oncology phase III trials: a comparison of the current status versus the expectations of the EORTC members.Ann Oncol. 2016 Jan;27(1):192-8. doi: 10.1093/annonc/mdv485. Epub 2015 Oct 19. Ann Oncol. 2016. PMID: 26483049
-
Health-related quality of life assessment in contemporary phase III trials in advanced colorectal cancer.Cancer Treat Rev. 2016 Nov;50:194-199. doi: 10.1016/j.ctrv.2016.09.015. Epub 2016 Sep 30. Cancer Treat Rev. 2016. PMID: 27718458 Review.
-
Deficiencies in health-related quality-of-life assessment and reporting: a systematic review of oncology randomized phase III trials published between 2012 and 2016.Ann Oncol. 2018 Dec 1;29(12):2288-2295. doi: 10.1093/annonc/mdy449. Ann Oncol. 2018. PMID: 30304498
Cited by
-
Price negotiation and pricing of anticancer drugs in China: An observational study.PLoS Med. 2024 Jan 2;21(1):e1004332. doi: 10.1371/journal.pmed.1004332. eCollection 2024 Jan. PLoS Med. 2024. PMID: 38166148 Free PMC article.
-
Clinician and Patient Reporting of Symptomatic Adverse Events in Cancer Clinical Trials: Using CTCAE and PRO-CTCAE® to Provide Two Distinct and Complementary Perspectives.Patient Relat Outcome Meas. 2022 Dec 8;13:249-258. doi: 10.2147/PROM.S256567. eCollection 2022. Patient Relat Outcome Meas. 2022. PMID: 36524232 Free PMC article. Review.
-
Patient experiences with patient-reported outcome measures in metastatic breast cancer trials: qualitative interviews.J Patient Rep Outcomes. 2022 Jun 3;6(1):60. doi: 10.1186/s41687-022-00460-z. J Patient Rep Outcomes. 2022. PMID: 35657533 Free PMC article.
-
Polyester Polymeric Nanoparticles as Platforms in the Development of Novel Nanomedicines for Cancer Treatment.Cancers (Basel). 2021 Jul 6;13(14):3387. doi: 10.3390/cancers13143387. Cancers (Basel). 2021. PMID: 34298604 Free PMC article. Review.
References
-
- Tannock IF, Amir E, Booth CM, et al. Relevance of randomised controlled trials in oncology. Lancet Oncol. 2016;17:e560‐e567. - PubMed
-
- Rock EP, Kennedy DL, Furness MH, Pierce WF, Pazdur R, Burke LB. Patient‐reported outcomes supporting anticancer product approvals. J Clin Oncol. 2007;25:5094‐5099. - PubMed
-
- Kluetz PG, O'Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19:e267‐e274. - PubMed
-
- Scoggins JF, Patrick DL. The use of patient‐reported outcomes instruments in registered clinical trials: evidence from ClinicalTrials.gov. Contemp Clin Trials. 2009;30:289‐292. - PMC - PubMed
-
- Vodicka E, Kim K, Devine EB, Gnanasakthy A, Scoggins JF, Patrick DL. Inclusion of patient‐reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007–2013). Contemp Clin Trials. 2015;43:1‐9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical