Loss of the ER membrane protein complex subunit Emc3 leads to retinal bipolar cell degeneration in aged mice
- PMID: 32886670
- PMCID: PMC7473584
- DOI: 10.1371/journal.pone.0238435
Loss of the ER membrane protein complex subunit Emc3 leads to retinal bipolar cell degeneration in aged mice
Abstract
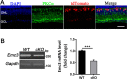
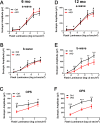
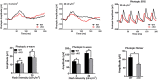
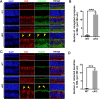
The endoplasmic reticulum (ER) membrane protein complex (EMC) is a conserved protein complex involved in inserting the transmembrane domain of membrane proteins into membranes in the ER. EMC3 is an essential component of EMC and is important for rhodopsin synthesis in photoreceptor cells. However, the in vivo function of Emc3 in bipolar cells (BCs) has not been determined. To explore the role of Emc3 in BCs, we generated a BC-specific Emc3 knockout mouse model (named Emc3 cKO) using the Purkinje cell protein 2 (Pcp2) Cre line. Although normal electroretinography (ERG) b-waves were observed in Emc3 cKO mice at 6 months of age, Emc3 cKO mice exhibited reduced b-wave amplitudes at 12 months of age, as determined by scotopic and photopic ERG, and progressive death of BCs, whereas the ERG a-wave amplitudes were preserved. PKCa staining of retinal cryosections from Emc3 cKO mice revealed death of rod BCs. Loss of Emc3 led to the presence of the synaptic protein mGLuR6 in the outer nuclear layer (ONL). Immunostaining analysis of presynaptic protein postsynaptic density protein 95 (PSD95) revealed rod terminals retracted to the ONL in Emc3 cKO mice at 12 months of age. In addition, deletion of Emc3 resulted in elevated glial fibrillary acidic protein, indicating reactive gliosis in the retina. Our data demonstrate that loss of Emc3 in BCs leads to decreased ERG response, increased astrogliosis and disruption of the retinal inner nuclear layer in mice of 12 months of age. Taken together, our studies indicate that Emc3 is not required for the development of BCs but is important for long-term survival of BCs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Tmem30a deficiency leads to retinal rod bipolar cell degeneration.J Neurochem. 2019 Feb;148(3):400-412. doi: 10.1111/jnc.14643. Epub 2019 Jan 15. J Neurochem. 2019. PMID: 30548540
-
EMC3 Is Essential for Retinal Organization and Neurogenesis During Mouse Retinal Development.Invest Ophthalmol Vis Sci. 2021 Feb 1;62(2):31. doi: 10.1167/iovs.62.2.31. Invest Ophthalmol Vis Sci. 2021. PMID: 33605987 Free PMC article.
-
Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery.Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3677-86. doi: 10.1167/iovs.07-1071. Invest Ophthalmol Vis Sci. 2008. PMID: 18660429 Free PMC article.
-
[Animal models of human retinal and optic nerve diseases analysed using electroretinography].Nippon Ganka Gakkai Zasshi. 2010 Mar;114(3):248-78, discussion 279. Nippon Ganka Gakkai Zasshi. 2010. PMID: 20387538 Review. Japanese.
-
The Function, Structure, and Origins of the ER Membrane Protein Complex.Annu Rev Biochem. 2022 Jun 21;91:651-678. doi: 10.1146/annurev-biochem-032620-104553. Epub 2022 Mar 14. Annu Rev Biochem. 2022. PMID: 35287476 Review.
Cited by
-
Characterization of Retinal VIP-Amacrine Cell Development During the Critical Period.Cell Mol Neurobiol. 2024 Feb 5;44(1):19. doi: 10.1007/s10571-024-01452-x. Cell Mol Neurobiol. 2024. PMID: 38315298 Free PMC article.
-
Exome sequencing in retinal dystrophy patients reveals a novel candidate gene ER membrane protein complex subunit 3.Heliyon. 2023 Sep 14;9(9):e20146. doi: 10.1016/j.heliyon.2023.e20146. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809982 Free PMC article.
-
Cellular stress signaling and the unfolded protein response in retinal degeneration: mechanisms and therapeutic implications.Mol Neurodegener. 2022 Mar 28;17(1):25. doi: 10.1186/s13024-022-00528-w. Mol Neurodegener. 2022. PMID: 35346303 Free PMC article. Review.
-
The endoplasmic reticulum membrane complex promotes proteostasis of GABAA receptors.iScience. 2022 Jul 13;25(8):104754. doi: 10.1016/j.isci.2022.104754. eCollection 2022 Aug 19. iScience. 2022. PMID: 35938049 Free PMC article.
-
Cell Type-Selective Loss of Peroxisomal β-Oxidation Impairs Bipolar Cell but Not Photoreceptor Survival in the Retina.Cells. 2022 Jan 4;11(1):161. doi: 10.3390/cells11010161. Cells. 2022. PMID: 35011723 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

