Memory CD73+IgM+ B cells protect against Plasmodium yoelii infection and express Granzyme B
- PMID: 32886698
- PMCID: PMC7473529
- DOI: 10.1371/journal.pone.0238493
Memory CD73+IgM+ B cells protect against Plasmodium yoelii infection and express Granzyme B
Abstract
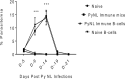
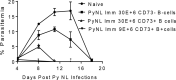
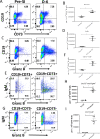
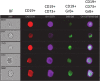
To better understand anti-malaria protective immune responses, we examined the cellular mechanisms that govern protective immunity in a murine Plasmodium yoelii 17X NL (PyNL) re-infection model. Initially, we confirmed that immune B cells generated during a primary PyNL infection were largely responsible for protection from a second PyNL infection. Using the previously identified memory B cell markers CD80, PD-L2, and CD73, we found an increase in the frequency of CD80-PD-L2-CD73+ B cells up to 55 days after a primary PyNL infection and at 4-6 days following a second PyNL infection. Moreover, injection of enriched immune CD19+CD73+ B cells into nonimmune mice were significantly more protective against a PyNL infection than CD73- B cells. Interestingly, a substantial fraction of these CD73+ B cells also expressed IgM and granzyme B, a biomolecule that has been increasingly associated with protective responses against malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- WHO. World Malaria Report 2019. https://www.who.int/malaria/publications/world-malaria-report-2019/en/
-
- Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961; (192):733–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

