Corneal dysfunction precedes the onset of hyperglycemia in a mouse model of diet-induced obesity
- PMID: 32886728
- PMCID: PMC7473521
- DOI: 10.1371/journal.pone.0238750
Corneal dysfunction precedes the onset of hyperglycemia in a mouse model of diet-induced obesity
Abstract
Purpose: The purpose of this study was to use a mouse model of diet-induced obesity to determine if corneal dysfunction begins prior to the onset of sustained hyperglycemia and if the dysfunction is ameliorated by diet reversal.
Methods: Six-week-old male C57BL/6 mice were fed a high fat diet (HFD) or a normal diet (ND) for 5-15 weeks. Diet reversal (DiR) mice were fed a HFD for 5 weeks, followed by a ND for 5 or 10 weeks. Corneal sensitivity was determined using aesthesiometry. Corneal cytokine expression was analyzed using a 32-plex Luminex assay. Excised corneas were prepared for immunofluorescence microscopy to evaluate diet-induced changes and wound healing. For wounding studies, mice were fed a HFD or a ND for 10 days prior to receiving a central 2mm corneal abrasion.
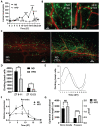
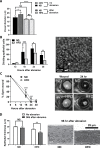
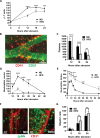
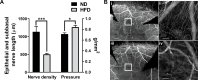
Results: After 10 days of HFD consumption, corneal sensitivity declined. By 10 weeks, expression of corneal inflammatory mediators increased and nerve density declined. While diet reversal restored nerve density and sensitivity, the corneas remained in a heightened inflammatory state. After 10 days on the HFD, corneal circadian rhythms (limbal neutrophil accumulation, epithelial cell division and Rev-erbα expression) were blunted. Similarly, leukocyte recruitment after wounding was dysregulated and accompanied by delays in wound closure and nerve recovery.
Conclusion: In the mouse, obesogenic diet consumption results in corneal dysfunction that precedes the onset of sustained hyperglycemia. Diet reversal only partially ameliorated this dysfunction, suggesting a HFD diet may have a lasting negative impact on corneal health that is resistant to dietary therapeutic intervention.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

