Sequential response-driven bortezomib-based therapy followed by autologous stem cell transplant in AL amyloidosis
- PMID: 32886751
- PMCID: PMC7479942
- DOI: 10.1182/bloodadvances.2020002219
Sequential response-driven bortezomib-based therapy followed by autologous stem cell transplant in AL amyloidosis
Abstract
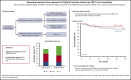
Autologous stem cell transplant (ASCT) is highly effective in selected patients with light chain (AL) amyloidosis. Bortezomib, preceding or following ASCT, improves responses. Satisfactory responses, including at least a partial response, very good partial response (VGPR) with organ response, or complete response, can be observed after induction therapy alone. We report 139 patients treated upfront with cyclophosphamide/bortezomib/dexamethasone (CyBorD), followed by ASCT only if response was unsatisfactory. Only 1 treatment-related death was observed. After CyBorD, hematologic response (HR) rate was 68% (VGPR or better, 51%), with 45% satisfactory responses. Transplant was performed in 55 (40%) subjects and resulted in an 80% HR rate (65% ≥ VGPR). Five-year survival was 86% and 84% in patients treated with ASCT or CyBorD alone, respectively (P = .438). Also, 6- and 12- month landmark analyses did not show differences in survival. Duration of response was not different in the 2 groups (60 vs 49 months; P = .670). Twenty-one (15%) patients with an unsatisfactory response to CyBorD could not undergo ASCT because of ineligibility or refusal; instead, they received rescue chemotherapy, with HR in 38% of cases and 51% 5-year survival. This sequential response-driven approach, offering ASCT to patients who do not attain satisfactory response to upfront CyBorD, is very safe and effective in AL amyloidosis.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.P. receives honoraria from Janssen Pharmaceuticals, honoraria and travel support from Prothena, and travel support from Celgene. P.M. receives speaker honoraria from Pfizer and Jansen Pharmaceuticals and travel support from Celgene. M.N. receives honoraria from Janssen Pharmaceuticals. G.M. is a consultant for Millennium Pharmaceuticals Inc., Pfizer, and Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures

References
-
- Gertz MA, Lacy MQ, Dispenzieri A, et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48(4):557-561. - PubMed
-
- Sanchorawala V, Sun F, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015;126(20):2345-2347. - PubMed
-
- Sidiqi MH, Aljama MA, Buadi FK, et al. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36(13):1323-1329. - PubMed
-
- Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612-615. - PubMed

