FOLFIRINOX De-Escalation in Advanced Pancreatic Cancer: A Multicenter Real-Life Study
- PMID: 32886823
- PMCID: PMC7648331
- DOI: 10.1634/theoncologist.2020-0577
FOLFIRINOX De-Escalation in Advanced Pancreatic Cancer: A Multicenter Real-Life Study
Abstract
Background: Our study describes the feasibility and efficacy of a first-line FOLFIRINOX (5-fluorouracil [5FU], folinic acid, irinotecan, and oxaliplatin) induction chemotherapy (CT) followed by de-escalation as a maintenance strategy for advanced pancreatic cancer.
Materials and methods: This multicenter retrospective study was conducted from January 2011 to December 2018. FOLFIRINOX de-escalation was defined as stopping oxaliplatin and/or irinotecan after at least four cycles of FOLFIRINOX, without evidence of disease progression. Maintenance schedules were fluoropyrimidine monotherapy (intravenous or oral [capecitabine]), FOLFOX (5FU, oxaliplatin), or FOLFIRI (5FU, irinotecan). Primary endpoint was overall survival (OS). Secondary endpoints were first progression-free survival (PFS1), second progression-free survival (PFS2), and toxicity.
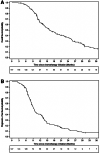
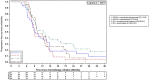
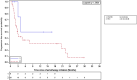
Results: Among 321 patients treated with FOLFIRINOX, 147 (45.8%) were included. Median OS was 16.1 months (95% confidence interval [CI], 13.7-20.3) and median PFS1 was 9.4 months (95% CI, 8.5-10.4). The preferred maintenance regimen was FOLFIRI in 66 (45%) patients versus 5FU monotherapy in 52 (35%) and FOLFOX in 25 (17%) patients. Among 118 patients who received maintenance CT with FOLFIRI or 5FU, there was no difference in PFS1 (median, 9.0 vs. 10.1 months, respectively; p = .33) or OS (median, 16.6 vs. 18.7 months; p = .86) between the two maintenance regimens. Reintroduction of FOLFIRINOX was performed in 20.2% of patients, with a median PFS2 of 2.8 months (95% CI, 2.0-22.3). The rates of grade 3-4 toxicity were significantly higher with FOLFIRI maintenance CT than with 5FU (41% vs. 22%; p = .03), especially for neuropathy (73% vs. 9%).
Conclusion: 5FU monotherapy maintenance appeared to be as effective as FOLFIRI, in a FOLFIRINOX de-escalation strategy, which is largely used in France.
Implications for practice: FOLFIRINOX de-escalation and maintenance is a feasible strategy in advanced pancreatic cancer that decreases chemotherapy toxicity to improve both survival and quality of life. Survivals in patients with maintenance therapy are clinically meaningful. Fluoropyrimidine monotherapy maintenance seems to be as efficient as FOLFIRI and should be a reference arm in future pancreatic cancer maintenance trials.
Keywords: Advanced pancreatic cancer; FOLFIRINOX; Maintenance treatment; Quality of life; Real-life study.
© AlphaMed Press 2020.
Conflict of interest statement
Figures

References
-
- Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol 2016;55:1158‐1160. - PubMed
-
- Rahib L, Smith BD, Aizenberg R et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913‐2921. - PubMed
-
- Neuzillet C, Tijeras‐Raballand A, Bourget P et al. State of the art and future directions of pancreatic ductal adenocarcinoma therapy. Pharmacol Ther 2015;155:80‐104. - PubMed
-
- Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817‐1825. - PubMed
-
- Gourgou‐Bourgade S, Bascoul‐Mollevi C, Desseigne F et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23‐29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

