Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury
- PMID: 32887360
- PMCID: PMC7563654
- DOI: 10.3390/cells9092020
Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury
Abstract
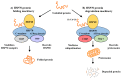
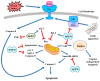
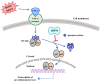
The 70 kDa heat shock protein (HSP70) is a stress-inducible protein that has been shown to protect the brain from various nervous system injuries. It allows cells to withstand potentially lethal insults through its chaperone functions. Its chaperone properties can assist in protein folding and prevent protein aggregation following several of these insults. Although its neuroprotective properties have been largely attributed to its chaperone functions, HSP70 may interact directly with proteins involved in cell death and inflammatory pathways following injury. Through the use of mutant animal models, gene transfer, or heat stress, a number of studies have now reported positive outcomes of HSP70 induction. However, these approaches are not practical for clinical translation. Thus, pharmaceutical compounds that can induce HSP70, mostly by inhibiting HSP90, have been investigated as potential therapies to mitigate neurological disease and lead to neuroprotection. This review summarizes the neuroprotective mechanisms of HSP70 and discusses potential ways in which this endogenous therapeutic molecule could be practically induced by pharmacological means to ultimately improve neurological outcomes in acute neurological disease.
Keywords: brain injury; chaperone neuroprotection; heat shock protein 70; pharmacological induction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke.Expert Opin Ther Targets. 2018 Mar;22(3):191-199. doi: 10.1080/14728222.2018.1439477. Epub 2018 Feb 15. Expert Opin Ther Targets. 2018. PMID: 29421932 Free PMC article. Review.
-
Pharmacological induction of the 70-kDa heat shock protein protects against brain injury.Neuroscience. 2015 Jan 22;284:912-919. doi: 10.1016/j.neuroscience.2014.11.010. Epub 2014 Nov 13. Neuroscience. 2015. PMID: 25446362 Free PMC article.
-
Regulation of ClC-2 Chloride Channel Proteostasis by Molecular Chaperones: Correction of Leukodystrophy-Associated Defect.Int J Mol Sci. 2021 May 30;22(11):5859. doi: 10.3390/ijms22115859. Int J Mol Sci. 2021. PMID: 34070744 Free PMC article.
-
17-AAG post-treatment ameliorates memory impairment and hippocampal CA1 neuronal autophagic death induced by transient global cerebral ischemia.Brain Res. 2015 Jun 12;1610:80-8. doi: 10.1016/j.brainres.2015.03.051. Epub 2015 Apr 7. Brain Res. 2015. PMID: 25858486
-
Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury.Inflammopharmacology. 2012 Jun;20(3):177-85. doi: 10.1007/s10787-011-0115-3. Epub 2012 Jan 13. Inflammopharmacology. 2012. PMID: 22246599 Review.
Cited by
-
Marine Bioprospecting: Enzymes and Stress Proteins from the Sea Anemones Anthopleura dowii and Lebrunia neglecta.Mar Drugs. 2023 Dec 23;22(1):12. doi: 10.3390/md22010012. Mar Drugs. 2023. PMID: 38248637 Free PMC article.
-
Decreased Levels of Chaperones in Mucopolysaccharidoses and Their Elevation as a Putative Auxiliary Therapeutic Approach.Pharmaceutics. 2023 Feb 20;15(2):704. doi: 10.3390/pharmaceutics15020704. Pharmaceutics. 2023. PMID: 36840025 Free PMC article.
-
Genome wide and evolutionary analysis of heat shock protein 70 proteins in tomato and their role in response to heat and drought stress.Mol Biol Rep. 2022 Dec;49(12):11229-11241. doi: 10.1007/s11033-022-07734-1. Epub 2022 Jul 4. Mol Biol Rep. 2022. PMID: 35788950
-
Heat Shock Protein 70 in Penile Neurovascular Regeneration Requires Cystathionine Gamma-Lyase.World J Mens Health. 2022 Oct;40(4):580-599. doi: 10.5534/wjmh.210249. Epub 2022 May 19. World J Mens Health. 2022. PMID: 36047068 Free PMC article.
-
Inhalative as well as Intravenous Administration of H2S Provides Neuroprotection after Ischemia and Reperfusion Injury in the Rats' Retina.Int J Mol Sci. 2022 May 15;23(10):5519. doi: 10.3390/ijms23105519. Int J Mol Sci. 2022. PMID: 35628328 Free PMC article.
References
-
- States B.A., Honkaniemi J., Weinstein P.R., Sharp F.R. DNA fragmentation and hsp70 protein induction in hippocampus and cortex occurs in separate neurons following permanent middle cerebral artery occlusions. J. Cereb. Blood Flow Metab. 1996;16:1165–1175. doi: 10.1097/00004647-199611000-00011. - DOI - PubMed

