SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19
- PMID: 32887634
- PMCID: PMC7471641
- DOI: 10.1186/s13045-020-00954-7
SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19
Abstract
Background: Critically ill patients diagnosed with COVID-19 may develop a pro-thrombotic state that places them at a dramatically increased lethal risk. Although platelet activation is critical for thrombosis and is responsible for the thrombotic events and cardiovascular complications, the role of platelets in the pathogenesis of COVID-19 remains unclear.
Methods: Using platelets from healthy volunteers, non-COVID-19 and COVID-19 patients, as well as wild-type and hACE2 transgenic mice, we evaluated the changes in platelet and coagulation parameters in COVID-19 patients. We investigated ACE2 expression and direct effect of SARS-CoV-2 virus on platelets by RT-PCR, flow cytometry, Western blot, immunofluorescence, and platelet functional studies in vitro, FeCl3-induced thrombus formation in vivo, and thrombus formation under flow conditions ex vivo.
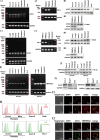
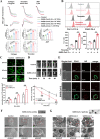
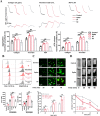
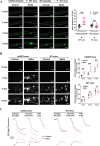
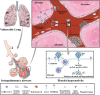
Results: We demonstrated that COVID-19 patients present with increased mean platelet volume (MPV) and platelet hyperactivity, which correlated with a decrease in overall platelet count. Detectable SARS-CoV-2 RNA in the blood stream was associated with platelet hyperactivity in critically ill patients. Platelets expressed ACE2, a host cell receptor for SARS-CoV-2, and TMPRSS2, a serine protease for Spike protein priming. SARS-CoV-2 and its Spike protein directly enhanced platelet activation such as platelet aggregation, PAC-1 binding, CD62P expression, α granule secretion, dense granule release, platelet spreading, and clot retraction in vitro, and thereby Spike protein enhanced thrombosis formation in wild-type mice transfused with hACE2 transgenic platelets, but this was not observed in animals transfused with wild-type platelets in vivo. Further, we provided evidence suggesting that the MAPK pathway, downstream of ACE2, mediates the potentiating role of SARS-CoV-2 on platelet activation, and that platelet ACE2 expression decreases following SARS-COV-2 stimulation. SARS-CoV-2 and its Spike protein directly stimulated platelets to facilitate the release of coagulation factors, the secretion of inflammatory factors, and the formation of leukocyte-platelet aggregates. Recombinant human ACE2 protein and anti-Spike monoclonal antibody could inhibit SARS-CoV-2 Spike protein-induced platelet activation.
Conclusions: Our findings uncovered a novel function of SARS-CoV-2 on platelet activation via binding of Spike to ACE2. SARS-CoV-2-induced platelet activation may participate in thrombus formation and inflammatory responses in COVID-19 patients.
Keywords: ACE2; COVID-19; Platelet activation; TMPRSS2; Thrombosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and Cardiovascular Disease. Circulation. 2020;141(20):1648–1655. - PubMed
-
- Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81803522/Young Scientists Fund/International
- 81900378/Young Scientists Fund/International
- 81970305/National Natural Science Foundation of China (CN)/International
- 81770137/National Natural Science Foundation of China/International
- 81573423/National Natural Science Foundation of China/International
- 81903603/National Natural Science Foundation of China/International
- 81400323/National Natural Science Foundation of China/International
- 201100310900/Emergency Prevention and Control of COVID-19 Project of Henan Province/International
- 2020M672292/Postdoctoral Research Foundation of China/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

