tRNA-derived RNA fragments in cancer: current status and future perspectives
- PMID: 32887641
- PMCID: PMC7487644
- DOI: 10.1186/s13045-020-00955-6
tRNA-derived RNA fragments in cancer: current status and future perspectives
Abstract
Non-coding RNAs (ncRNAs) have been the focus of many studies over the last few decades, and their fundamental roles in human diseases have been well established. Transfer RNAs (tRNAs) are housekeeping ncRNAs that deliver amino acids to ribosomes during protein biosynthesis. tRNA fragments (tRFs) are a novel class of small ncRNAs produced through enzymatic cleavage of tRNAs and have been shown to play key regulatory roles similar to microRNAs. Development and application of high-throughput sequencing technologies has provided accumulating evidence of dysregulated tRFs in cancer. Aberrant expression of tRFs has been found to participate in cell proliferation, invasive metastasis, and progression in several human malignancies. These newly identified functional tRFs also have great potential as new biomarkers and therapeutic targets for cancer treatment. In this review, we focus on the major biological functions of tRFs including RNA silencing, translation regulation, and epigenetic regulation; summarize recent research on the roles of tRFs in different types of cancer; and discuss the potential of using tRFs as clinical biomarkers for cancer diagnosis and prognosis and as therapeutic targets for cancer treatment.
Keywords: Biomarkers; Cancer; Epigenetic regulation; RNA silencing; Translation regulation; tRNA-derived fragments.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
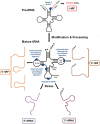
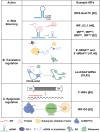

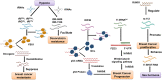
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

