Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: An evidence-based clinical practice guideline (updated version)
- PMID: 32887670
- PMCID: PMC7472403
- DOI: 10.1186/s40779-020-00270-8
Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: An evidence-based clinical practice guideline (updated version)
Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of a rapidly spreading illness, coronavirus disease 2019 (COVID-19), affecting more than seventeen million people around the world. Diagnosis and treatment guidelines for clinicians caring for patients are needed. In the early stage, we have issued "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)"; now there are many direct evidences emerged and may change some of previous recommendations and it is ripe for develop an evidence-based guideline. We formed a working group of clinical experts and methodologists. The steering group members proposed 29 questions that are relevant to the management of COVID-19 covering the following areas: chemoprophylaxis, diagnosis, treatments, and discharge management. We searched the literature for direct evidence on the management of COVID-19, and assessed its certainty generated recommendations using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. Recommendations were either strong or weak, or in the form of ungraded consensus-based statement. Finally, we issued 34 statements. Among them, 6 were strong recommendations for, 14 were weak recommendations for, 3 were weak recommendations against and 11 were ungraded consensus-based statement. They covered topics of chemoprophylaxis (including agents and Traditional Chinese Medicine (TCM) agents), diagnosis (including clinical manifestations, reverse transcription-polymerase chain reaction (RT-PCR), respiratory tract specimens, IgM and IgG antibody tests, chest computed tomography, chest x-ray, and CT features of asymptomatic infections), treatments (including lopinavir-ritonavir, umifenovir, favipiravir, interferon, remdesivir, combination of antiviral drugs, hydroxychloroquine/chloroquine, interleukin-6 inhibitors, interleukin-1 inhibitors, glucocorticoid, qingfei paidu decoction, lianhua qingwen granules/capsules, convalescent plasma, lung transplantation, invasive or noninvasive ventilation, and extracorporeal membrane oxygenation (ECMO)), and discharge management (including discharge criteria and management plan in patients whose RT-PCR retesting shows SARS-CoV-2 positive after discharge). We also created two figures of these recommendations for the implementation purpose. We hope these recommendations can help support healthcare workers caring for COVID-19 patients.
Keywords: COVID-19; Chemoprophylaxis; Diagnosis; Discharge management; Recommendation; SARS-CoV-2; Traditional Chinese medicine; guideline; Treatment.
Conflict of interest statement
No one reported having stock, being a consultant paid by companies or receiving research funding from companies that have an interest in the guideline. Zhen-Shun Cheng reported participating in or hosting a study of Remdesivir for COVID-19. Ya-Dong Gao reported participating in or hosting two studies of Enteric capsule with diammonium glycyrrhizate combined with vitamin C and hydroxychloroquine for COVID-19. Jian Xia reported participating in or hosting two studies of ECOM treatment for critical patients and a prediction model for COVID-19 prognosis. Qi-Wen Yang reported participating in or hosting a study of clinical evaluation of rapid nucleic acid detection kit. Xiao-Chun Zhang reported participating in or hosting a study of COVID-19 technical research and integrated application projects. Yu-Feng Yuan reported participating in or hosting three studies of Clinical course and prognosis, TCM treatment and rapid disinfection of medical items for COVID-19. Xun-Tao Yin reported participating in or hosting CT imaging diagnosis for COVID-19. Zhui Yu reported participating in or hosting study of treatment for critical COVID-19 patients. Hong-Jun Li reported participating in or hosting study of machine learning for radiology of COVID-19. Xing-Huan Wang leading the favipiravir randomized controlled trial and it was supported by the National Key Research and Development Program of China (2020YFC0844400); Xing-Huan Wang, Xian-Tao Zeng and Ying-Hui Jin reported research projects involving infection of healthcare workers during this epidemic, which was supported by Special Project for Emergency of Hubei Province (2020FCA008). No other disclosures were reported.
Figures
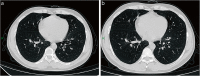

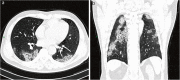
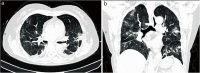




References
-
- WHO. WHO coronavirus disease (covid-19) dashboard. https://covid19.who.int/. Accessed 31 July 2020.
-
- Jin YH, Li HJ, Zhan QY, Peng ZY, F YY, Cai L, et al. Evidence-based Chinese expert recommendations on drug prevention, diagnosis, treatment, and discharge management of COVID-19: a protocol. Yixue Xinzhi Zazhi. 2020;30(3):209–226.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

