Immunological considerations for COVID-19 vaccine strategies
- PMID: 32887954
- PMCID: PMC7472682
- DOI: 10.1038/s41577-020-00434-6
Immunological considerations for COVID-19 vaccine strategies
Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most formidable challenge to humanity in a century. It is widely believed that prepandemic normalcy will never return until a safe and effective vaccine strategy becomes available and a global vaccination programme is implemented successfully. Here, we discuss the immunological principles that need to be taken into consideration in the development of COVID-19 vaccine strategies. On the basis of these principles, we examine the current COVID-19 vaccine candidates, their strengths and potential shortfalls, and make inferences about their chances of success. Finally, we discuss the scientific and practical challenges that will be faced in the process of developing a successful vaccine and the ways in which COVID-19 vaccine strategies may evolve over the next few years.
Conflict of interest statement
The authors declare no competing interests.
Figures
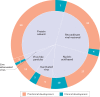
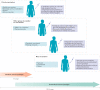
References
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. WHOhttps://covid19.who.int/ (2020).
-
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. WHOhttps://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

