Application of Pharmacokinetic-Pharmacodynamic Modeling to Inform Translation of In Vitro NaV1.7 Inhibition to In Vivo Pharmacological Response in Non-human Primate
- PMID: 32888082
- PMCID: PMC7473964
- DOI: 10.1007/s11095-020-02914-9
Application of Pharmacokinetic-Pharmacodynamic Modeling to Inform Translation of In Vitro NaV1.7 Inhibition to In Vivo Pharmacological Response in Non-human Primate
Abstract
Purpose: This work describes a staged approach to the application of pharmacokinetic-pharmacodynamic (PK-PD) modeling in the voltage-gated sodium ion channel (NaV1.7) inhibitor drug discovery effort to address strategic questions regarding in vitro to in vivo translation of target modulation.
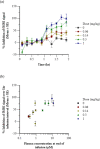
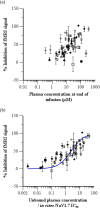
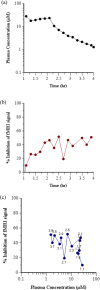
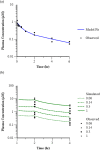
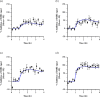
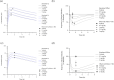
Methods: PK-PD analysis was applied to data from a functional magnetic resonance imaging (fMRI) technique to non-invasively measure treatment mediated inhibition of olfaction signaling in non-human primates (NHPs). Initial exposure-response was evaluated using single time point data pooled across 27 compounds to inform on in vitro to in vivo correlation (IVIVC). More robust effect compartment PK-PD modeling was conducted for a subset of 10 compounds with additional PD and PK data to characterize hysteresis.
Results: The pooled compound exposure-response facilitated an early exploration of IVIVC with a limited dataset for each individual compound, and it suggested a 2.4-fold in vitro to in vivo scaling factor for the NaV1.7 target. Accounting for hysteresis with an effect compartment PK-PD model as compounds advanced towards preclinical development provided a more robust determination of in vivo potency values, which resulted in a statistically significant positive IVIVC with a slope of 1.057 ± 0.210, R-squared of 0.7831, and p value of 0.006. Subsequent simulations with the PK-PD model informed the design of anti-nociception efficacy studies in NHPs.
Conclusions: A staged approach to PK-PD modeling and simulation enabled integration of in vitro NaV1.7 potency, plasma protein binding, and pharmacokinetics to describe the exposure-response profile and inform future study design as the NaV1.7 inhibitor effort progressed through drug discovery.
Keywords: NaV1.7; PK-PD; fMRI; nociception; olfaction.
Figures


References
-
- Wong H, Bohnert T, Damian-Lordache V, Gibson C, Hsu C-P, Krishnatry AS, et al. Translational pharmacokinetic-pharmacodynamic analysis in the pharmaceutical industry: an IQ consortium PK-PD discussion group perspective. Drug Discov Today. 2017;22:1447–1459. doi: 10.1016/j.drudis.2017.04.015. - DOI - PubMed
-
- Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street SD. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

