Sex Differences in Electrophysiological Properties of Mouse Medial Preoptic Area Neurons Revealed by In Vitro Whole-cell Recordings
- PMID: 32888180
- PMCID: PMC7870743
- DOI: 10.1007/s12264-020-00565-9
Sex Differences in Electrophysiological Properties of Mouse Medial Preoptic Area Neurons Revealed by In Vitro Whole-cell Recordings
Abstract
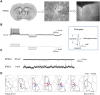
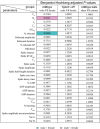
Despite extensive characterization of sex differences in the medial preoptic area (mPOA) of the hypothalamus, we know surprisingly little about whether or how male and female mPOA neurons differ electrophysiologically, especially in terms of neuronal firing and behavioral pattern generation. In this study, by performing whole-cell patch clamp recordings of the mPOA, we investigated the influences of sex, cell type, and gonadal hormones on the electrophysiological properties of mPOA neurons. Notably, we uncovered significant sex differences in input resistance (male > female) and in the percentage of neurons that displayed post-inhibitory rebound (male > female). Furthermore, we found that the current mediated by the T-type Ca2+ channel (IT), which is known to underlie post-inhibitory rebound, was indeed larger in male mPOA neurons. Thus, we have identified salient electrophysiological properties of mPOA neurons, namely IT and post-inhibitory rebound, that are male-biased and likely contribute to the sexually dimorphic display of behaviors.
Keywords: Electrophysiological properties; Post-inhibitory rebound; Sex differences; T-type calcium channels; mPOA.
Conflict of interest statement
The authors claim no conflict of interest.
Figures

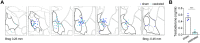

References
-
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 1999, 877: 242–257. - PubMed
-
- O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. - PubMed
-
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

