Longitudinal Measurements of Glucocerebrosidase activity in Parkinson's patients
- PMID: 32888397
- PMCID: PMC7545591
- DOI: 10.1002/acn3.51164
Longitudinal Measurements of Glucocerebrosidase activity in Parkinson's patients
Abstract
Objective: Reduction in glucocerebrosidase (GCase; encoded by GBA) enzymatic activity has been linked to Parkinson's disease (PD). Here, we correlated GCase activity and PD phenotype in the Parkinson's Progression Markers Initiative (PPMI) cohort.
Methods: We measured GCase activity in dried blood spots from 1559 samples of participants in the inception PPMI cohort, collected in four annual visits (from baseline visit to Year-3). Participants (PD, n = 392; controls, n = 175) were fully sequenced for GBA variants by means of genome-wide genotyping arrays, whole-exome sequencing, whole-genome sequencing, Sanger sequencing, and RNA-sequencing.
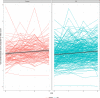
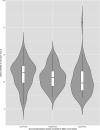
Results: Fifty-two PD participants (13.4%) and 13 (7.4%) controls carried a GBA variant. GBA status was strongly associated with GCase activity. Among noncarriers, GCase activity was similar between PD and controls. Among GBA p.E326K carriers (PD, n = 20; controls, n = 5), activity was significantly lower in PD carriers than control carriers (9.53 µmol/L/h vs. 11.68 µmol/L/h, P = 0.035). Glucocerebrosidase activity was moderately (r = 0.45) associated with white blood cell (WBC) count. Next, we divided the noncarriers with PD to tertiles based on WBC count-corrected enzymatic activity. Members of the lower tertile had higher MDS-Unified Parkinson's Disease Rating Scale motor score in the "off" medication examination at year-III exam. Longitudinal analyses demonstrated slight reduction of activity in samples collected earlier on in the study, likely because of longer storage time.
Interpretation: GCase activity is associated with GBA genotype, WBC count, and among p.E326K variant carriers, with PD status. Reduced activity may also be associated with worse phenotype but longer follow up is required to confirm this observation.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Pavlina Wolf, Kate Zhang, Ming Sum, Ruby Chiang, Karolina Helesicova and Sergio Pablo Sardi are/were employees of Sanofi during their work on the manuscript. All other authors have no conflict of interest related to this work.
Roy Alcalay is funded by the NIH, the Parkinson’s Foundation and the Michael. J. Fox Foundation. He received consultation fees from Sanofi, Roche, Janssen and Restorbio. Dr Gan‐Or is supported by the Fonds de recherche du Québec ‐ Santé (FRQS) Chercheurs‐boursiers award given in collaboration with Parkinson Quebec, and is a Parkinson Canada New Investigator awardee. He received consultancy fees from Lysosomal Therapeutics Inc. (LTI), Idorsia, Prevail Therapeutics, Inceptions Sciences (now Ventus), Denali, Ono Therapeutics and Deerfield. Tanya Simuni, MD has served as a consultant for Acadia, Abbvie, Accorda, Adamas, Allergan, Amneal, Anavex, Aptinyx, Blue Rock Therapeutics, Denali, General Electric (GE), Neuroderm, Neurocrine, Sanofi, Sinopia, Sunovion, TEVA, Takeda, Voyager, US World Meds, Parkinson’s Foundation, and the Michael J. Fox Foundation for Parkinson’s Research; Dr. Simuni has served as a speaker and received an honorarium from Acadia and Adamas; Dr Simuni is on the Scientific advisory board for Anavex, Neuroderm, Sanofi, and MJFF. Dr. Simuni has received research funding from the NINDS, Parkinson’s Foundation, MJFF, Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Abbvie, and IMPAX. Lana M. Chahine receives research support from the Michael J Fox Foundation, including for PPMI, has received travel payment from MJFF to MJFF conferences, is a paid consultant to MJFF, receives research support from the UPMC Competitive Medical Research Fund, is study site investigator for a study sponsored by Biogen, is a subinvestigator for a study sponsored by Voyager, and receives royalties from Wolters Kluwel (for book authorship). Cornelis Blauwendraat is an NIH employee and reports no disclosures.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
