Cortical Encoding of Manual Articulatory and Linguistic Features in American Sign Language
- PMID: 32888480
- PMCID: PMC7674262
- DOI: 10.1016/j.cub.2020.08.048
Cortical Encoding of Manual Articulatory and Linguistic Features in American Sign Language
Abstract
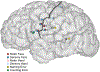
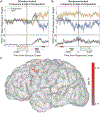
The fluent production of a signed language requires exquisite coordination of sensory, motor, and cognitive processes. Similar to speech production, language produced with the hands by fluent signers appears effortless but reflects the precise coordination of both large-scale and local cortical networks. The organization and representational structure of sensorimotor features underlying sign language phonology in these networks remains unknown. Here, we present a unique case study of high-density electrocorticography (ECoG) recordings from the cortical surface of profoundly deaf signer during awake craniotomy. While neural activity was recorded from sensorimotor cortex, the participant produced a large variety of movements in linguistic and transitional movement contexts. We found that at both single electrode and neural population levels, high-gamma activity reflected tuning for particular hand, arm, and face movements, which were organized along dimensions that are relevant for phonology in sign language. Decoding of manual articulatory features revealed a clear functional organization and population dynamics for these highly practiced movements. Furthermore, neural activity clearly differentiated linguistic and transitional movements, demonstrating encoding of language-relevant articulatory features. These results provide a novel and unique view of the fine-scale dynamics of complex and meaningful sensorimotor actions.
Keywords: deaf; direct electrical stimulation; electrocorticography; sensorimotor control; sign language.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



Comment in
-
Sign Language: How the Brain Represents Phonology without Sound.Curr Biol. 2020 Nov 16;30(22):R1361-R1363. doi: 10.1016/j.cub.2020.09.017. Curr Biol. 2020. PMID: 33202232 Free PMC article.
References
-
- Bellugi U, and Klima ES (1979). The Signs of Language (Harvard University Press; ).
-
- Emmorey K (2001). Language, cognition, and the brain: Insights from sign language research (Psychology Press; ).
-
- Sandler W, and Lillo-Martin D (2006). Sign language and linguistic universals (Cambridge University Press; ).
-
- Lillo-Martin D (1999). Modality effects and modularity in language acquisition: The acquisition of American Sign Language. Handb. Child Lang. Acquis 531, 567.

