Recurrent Processing Drives Perceptual Plasticity
- PMID: 32888488
- PMCID: PMC7658806
- DOI: 10.1016/j.cub.2020.08.016
Recurrent Processing Drives Perceptual Plasticity
Abstract
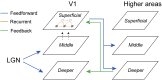
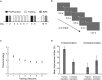
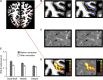
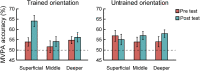
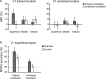
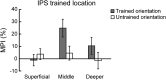
Learning and experience are critical for translating ambiguous sensory information from our environments to perceptual decisions. Yet evidence on how training molds the adult human brain remains controversial, as fMRI at standard resolution does not allow us to discern the finer scale mechanisms that underlie sensory plasticity. Here, we combine ultra-high-field (7T) functional imaging at sub-millimeter resolution with orientation discrimination training to interrogate experience-dependent plasticity across cortical depths that are known to support dissociable brain computations. We demonstrate that learning alters orientation-specific representations in superficial rather than middle or deeper V1 layers, consistent with recurrent plasticity mechanisms via horizontal connections. Further, learning increases feedforward rather than feedback layer-to-layer connectivity in occipito-parietal regions, suggesting that sensory plasticity gates perceptual decisions. Our findings reveal finer scale plasticity mechanisms that re-weight sensory signals to inform improved decisions, bridging the gap between micro- and macro-circuits of experience-dependent plasticity.
Keywords: experience-dependent plasticity; layer-to-layer functional connectivity; learning; perceptual decisions; ultra-high-field brain imaging; visual cortex.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

Comment in
-
Perceptual Learning: How Does the Visual Circuit Change through Experience?Curr Biol. 2020 Nov 2;30(21):R1309-R1311. doi: 10.1016/j.cub.2020.08.097. Curr Biol. 2020. PMID: 33142098
References
Publication types
MeSH terms
Grants and funding
- DH_/Department of Health/United Kingdom
- MR/M008983/1/MRC_/Medical Research Council/United Kingdom
- BB/P021255/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 205067/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- H012508/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

