Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations
- PMID: 32888493
- PMCID: PMC7480402
- DOI: 10.1016/j.cell.2020.06.045
Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations
Abstract
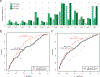
Most loci identified by GWASs have been found in populations of European ancestry (EUR). In trans-ethnic meta-analyses for 15 hematological traits in 746,667 participants, including 184,535 non-EUR individuals, we identified 5,552 trait-variant associations at p < 5 × 10-9, including 71 novel associations not found in EUR populations. We also identified 28 additional novel variants in ancestry-specific, non-EUR meta-analyses, including an IL7 missense variant in South Asians associated with lymphocyte count in vivo and IL-7 secretion levels in vitro. Fine-mapping prioritized variants annotated as functional and generated 95% credible sets that were 30% smaller when using the trans-ethnic as opposed to the EUR-only results. We explored the clinical significance and predictive value of trans-ethnic variants in multiple populations and compared genetic architecture and the effect of natural selection on these blood phenotypes between populations. Altogether, our results for hematological traits highlight the value of a more global representation of populations in genetic studies.
Keywords: interleukin-7, genetic architecture, fine-mapping, selective sweeps, polygenic trait score, phenome-wide association study.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests Competing financial interests are declared in Table S1F.
Figures




Comment in
-
Exploring the genetic basis of blood cell traits.Nat Rev Genet. 2020 Nov;21(11):650. doi: 10.1038/s41576-020-00289-6. Nat Rev Genet. 2020. PMID: 32943745 No abstract available.
References
-
- Brusselle GG, Provoost S, and Maes T (2016). Prostaglandin D2 receptor antagonism: a novel therapeutic option for eosinophilic asthma? Lancet Respir Med 4, 676–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 210561/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- R01 HL130733/HL/NHLBI NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- U01 HL130114/HL/NHLBI NIH HHS/United States
- U01 HL120393/HL/NHLBI NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- I01 BX003340/BX/BLRD VA/United States
- MR/R023484/1/MRC_/Medical Research Council/United Kingdom
- NC/P001076/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- R01 HL087652/HL/NHLBI NIH HHS/United States
- U01 HL137162/HL/NHLBI NIH HHS/United States
- R01 HL146500/HL/NHLBI NIH HHS/United States
- 001/WHO_/World Health Organization/International
- MR/S019669/1/MRC_/Medical Research Council/United Kingdom

