Limited effect of intramuscular epinephrine on cardiovascular parameters during peanut-induced anaphylaxis: An observational cohort study
- PMID: 32889224
- PMCID: PMC7794658
- DOI: 10.1016/j.jaip.2020.08.041
Limited effect of intramuscular epinephrine on cardiovascular parameters during peanut-induced anaphylaxis: An observational cohort study
Figures
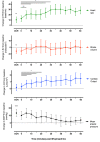

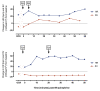
References
-
- Mullins R.J., Wainstein B.K., Barnes E.H., Liew W.K., Campbell D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46:1099–1110. - PubMed
-
- Soar J., Pumphrey R., Cant A., Clarke S., Corbett A., Dawson P. Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation. 2008;77:157–169. - PubMed
-
- Muraro A., Roberts G., Worm M., Bilò M.B., Brockow K., Fernández Rivas M. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026–1045. - PubMed
-
- Bautista E., Simons F.E.R., Simons K.J., Becker A.B., Duke K., Tillett M. Epinephrine fails to hasten hemodynamic recovery in fully developed canine anaphylactic shock. Int Arch Allergy Immunol. 2002;128:151–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

