Promising effects of tocilizumab in COVID-19: A non-controlled, prospective clinical trial
- PMID: 32889241
- PMCID: PMC7402206
- DOI: 10.1016/j.intimp.2020.106869
Promising effects of tocilizumab in COVID-19: A non-controlled, prospective clinical trial
Abstract
Background: The clinical presentation of SARS-CoV-2 infection ranges from mild symptoms to severe complications, including acute respiratory distress syndrome. In this syndrome, inflammatory cytokines are released after activation of the inflammatory cascade, with the predominant role of interleukin (IL)-6. The aim of this study was to evaluate the effects of tocilizumab, as an IL-6 antagonist, in patients with severe or critical SARS-CoV-2 infection.
Methods: In this prospective clinical trial, 76 patients with severe or critical SARS-CoV-2 infection were evaluated for eligibility, and ultimately, 42 patients were included. Tocilizumab was administered at a dose of 400 mg as a single dose via intravenous infusion. Primary outcomes included changes in oxygenation support, need for invasive mechanical ventilation, and death. Secondary outcomes included radiological changes in the lungs, IL-6 plasma levels, C-reactive protein levels, and adverse drug reactions. The data were analyzed using SPSS software.
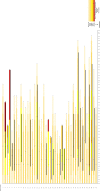
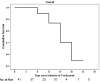
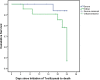
Results: Of the 42 included patients, 20 (48%) patients presented the severe infection stage and 22 (52%) were in the critical stage. The median age of patients was 56 years, and the median IL-6 level was 28.55 pg/mL. After tocilizumab administration, only 6 patients (14%) required invasive ventilation. Additionally, 35 patients (83.33%) showed clinical improvement. By day 28, a total of 7 patients died (6 patients in the critical stage and 1 patient in the severe stage). Neurological adverse effects were observed in 3 patients.
Conclusions: Based on the current results, tocilizumab may be a promising agent for patients with severe or critical SARS-CoV-2 infection, if promptly initiated during the severe stage.
Keywords: COVID-19; Coronavirus; Interleukin 6; SARS-CoV-2; Tocilizumab.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

