Genetic and clinical basis for two distinct subtypes of primary Sjögren's syndrome
- PMID: 32889544
- PMCID: PMC7850528
- DOI: 10.1093/rheumatology/keaa367
Genetic and clinical basis for two distinct subtypes of primary Sjögren's syndrome
Abstract
Objectives: Clinical presentation of primary Sjögren's syndrome (pSS) varies considerably. A shortage of evidence-based objective markers hinders efficient drug development and most clinical trials have failed to reach primary endpoints.
Methods: We performed a multicentre study to identify patient subgroups based on clinical, immunological and genetic features. Targeted DNA sequencing of 1853 autoimmune-related loci was performed. After quality control, 918 patients with pSS, 1264 controls and 107 045 single nucleotide variants remained for analysis. Replication was performed in 177 patients with pSS and 7672 controls.
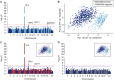
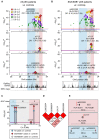
Results: We found strong signals of association with pSS in the HLA region. Principal component analysis of clinical data distinguished two patient subgroups defined by the presence of SSA/SSB antibodies. We observed an unprecedented high risk of pSS for an association in the HLA-DQA1 locus of odds ratio 6.10 (95% CI: 4.93, 7.54, P=2.2×10-62) in the SSA/SSB-positive subgroup, while absent in the antibody negative group. Three independent signals within the MHC were observed. The two most significant variants in MHC class I and II respectively, identified patients with a higher risk of hypergammaglobulinaemia, leukopenia, anaemia, purpura, major salivary gland swelling and lymphadenopathy. Replication confirmed the association with both MHC class I and II signals confined to SSA/SSB antibody positive pSS.
Conclusion: Two subgroups of patients with pSS with distinct clinical manifestations can be defined by the presence or absence of SSA/SSB antibodies and genetic markers in the HLA locus. These subgroups should be considered in clinical follow-up, drug development and trial outcomes, for the benefit of both subgroups.
Keywords: Sjögren’s syndrome; autoantibodies; autoimmunity; gene polymorphism.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Brito-Zeron P, Acar-Denizli N, Ng WF. et al. How immunological profile drives clinical phenotype of primary Sjögren’s syndrome at diagnosis: analysis of 10,500 patients (Sjögren Big Data Project). Clin Exp Rheumatol 2018;36(Suppl 112):102–12. - PubMed
-
- Kvarnstrom M, Ottosson V, Nordmark B. et al. Incident cases of primary Sjögren’s syndrome during a 5-year period in Stockholm County: a descriptive study of the patients and their characteristics. Scand J Rheumatol 2015;44:135–42. - PubMed
-
- Shiboski CH, Shiboski SC, Seror R. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. - PubMed
-
- Mariette X, Criswell LA.. Primary Sjögren’s syndrome. N Engl J Med 2018;378:931–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

