Reaction Pathways and Redox States in α-Selective Cobalt-Catalyzed Hydroborations of Alkynes
- PMID: 32889757
- PMCID: PMC7756293
- DOI: 10.1002/anie.202009625
Reaction Pathways and Redox States in α-Selective Cobalt-Catalyzed Hydroborations of Alkynes
Abstract
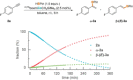
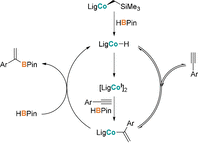
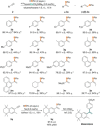
Cobalt(II) alkyl complexes supported by a monoanionic NNN pincer ligand are pre-catalysts for the regioselective hydroboration of terminal alkynes, yielding the Markovnikov products with α:β-(E) ratios of up to 97:3. A cobalt(II) hydride and a cobalt(II) vinyl complex appear to determine the main reaction pathway. In a background reaction the highly reactive hydrido species specifically converts to a coordinatively unsaturated cobalt(I) complex which was found to re-enter the main catalytic cycle.
Keywords: T-shaped complex; alkenyl boronates; alkynes; cobalt; pincer ligand.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cobalt-Catalyzed Markovnikov-Type Selective Hydroboration of Terminal Alkynes.Angew Chem Int Ed Engl. 2021 Jan 11;60(2):690-694. doi: 10.1002/anie.202012164. Epub 2020 Nov 10. Angew Chem Int Ed Engl. 2021. PMID: 32970357
-
An NNN-Pincer-Cobalt Complex Catalyzed Highly Markovnikov-Selective Alkyne Hydrosilylation.Org Lett. 2018 Oct 5;20(19):6265-6269. doi: 10.1021/acs.orglett.8b02746. Epub 2018 Sep 26. Org Lett. 2018. PMID: 30256120
-
Reactivity of a T-shaped cobalt(I) pincer-complex.Dalton Trans. 2021 May 25;50(20):6802-6810. doi: 10.1039/d1dt00277e. Dalton Trans. 2021. PMID: 34032245
-
(Z)-Selective Hydrosilylation and Hydroboration of Terminal Alkynes Enabled by Ruthenium Complexes with an N-Heterocyclic Carbene Ligand.Chem Rec. 2021 Dec;21(12):3429-3441. doi: 10.1002/tcr.202100083. Epub 2021 May 24. Chem Rec. 2021. PMID: 34028185 Review.
-
Cobalt-Pincer Complexes in Catalysis.Chemistry. 2019 Jan 2;25(1):122-143. doi: 10.1002/chem.201803016. Epub 2018 Dec 6. Chemistry. 2019. PMID: 30182374 Review.
Cited by
-
Exploring the Effect of Pincer Rigidity on Oxidative Addition Reactions with Cobalt(I) Complexes.Organometallics. 2023 Apr 24;42(8):708-718. doi: 10.1021/acs.organomet.3c00079. Epub 2023 Apr 5. Organometallics. 2023. PMID: 37223209 Free PMC article.
-
Tandem manganese catalysis for the chemo-, regio-, and stereoselective hydroboration of terminal alkynes: in situ precatalyst activation as a key to enhanced chemoselectivity.RSC Adv. 2024 Feb 13;14(8):5514-5523. doi: 10.1039/d3ra08747f. eCollection 2024 Feb 7. RSC Adv. 2024. PMID: 38352676 Free PMC article.
-
Stereoselective Semi-Hydrogenations of Alkynes by First-Row (3d) Transition Metal Catalysts.ChemCatChem. 2022 Oct 21;14(20):e202200886. doi: 10.1002/cctc.202200886. Epub 2022 Sep 15. ChemCatChem. 2022. PMID: 36632425 Free PMC article. Review.
-
Enhanced Dihydrogen Activation by Mononuclear Iridium(II) Compounds: A Mechanistic Study.Angew Chem Int Ed Engl. 2022 Aug 26;61(35):e202206831. doi: 10.1002/anie.202206831. Epub 2022 Jul 13. Angew Chem Int Ed Engl. 2022. PMID: 35737594 Free PMC article.
-
Cobalt-catalyzed branched selective hydroallylation of terminal alkynes.Nat Commun. 2022 Aug 3;13(1):4518. doi: 10.1038/s41467-022-32291-3. Nat Commun. 2022. PMID: 35922446 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

