Decline in Club Cell Secretory Proteins, Exosomes Induction and Immune Responses to Lung Self-antigens, Kα1 Tubulin and Collagen V, Leading to Chronic Rejection After Human Lung Transplantation
- PMID: 32890135
- PMCID: PMC7917153
- DOI: 10.1097/TP.0000000000003428
Decline in Club Cell Secretory Proteins, Exosomes Induction and Immune Responses to Lung Self-antigens, Kα1 Tubulin and Collagen V, Leading to Chronic Rejection After Human Lung Transplantation
Abstract
Background: Chronic lung allograft dysfunction (CLAD), is a major hurdle for long-term lung allograft survival after lung transplant and roughly 50% of lung transplant recipients (LTxRs) develop CLAD within 5 years. The mechanisms of CLAD development remain unknown. Donor-specific immune responses to HLA and lung self-antigens (SAgs) are vital to the pathogenesis of CLAD. Reduction in Club cell secretory protein (CCSP) has been reported in bronchoalveolar lavage (BAL) fluid samples from LTxRs with bronchiolitis obliterans syndrome (BOS). CCSP levels in BAL fluid and development of antibodies to lung SAgs in plasma were determined by ELISA. Cytokines in BAL fluid were analyzed by 30-plex Luminex panel. Exosomes from BAL fluid or plasma were analyzed for SAgs, natural killer (NK) cells markers, and cytotoxic molecules.
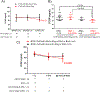
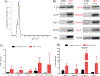
Results: We demonstrate that LTxRs with BOS have lower CCSP levels up to 9 months before BOS diagnosis. LTxRs with antibodies to SAgs 1-year posttransplant also developed DSA (43%) and had lower CCSP. BOS with lower CCSP also induced Interleukin-8 and reduced vascular endothelial growth factor. Exosomes from BOS contained increased SAgs, NK cells markers, and cytotoxic molecules.
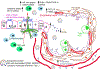
Conclusions: We conclude lower CCSP leads to inflammation, pro-inflammatory cytokine production, immune responses to HLA and SAgs, and induction of exosomes. For the first time, we demonstrate that CCSP loss results in exosome release from NK cells capable of stimulating innate and adaptive immunity posttransplant. This increases the risk of BOS, suggesting a role of NK cell exosomes in CLAD development.
Copyright © 2020 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures




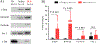

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

