Two serological approaches for detection of antibodies to SARS-CoV-2 in different scenarios: a screening tool and a point-of-care test
- PMID: 32890908
- PMCID: PMC7417941
- DOI: 10.1016/j.diagmicrobio.2020.115167
Two serological approaches for detection of antibodies to SARS-CoV-2 in different scenarios: a screening tool and a point-of-care test
Abstract
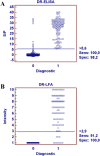
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected more than 8 million people worldwide, becoming a pandemic. Detecting antibodies against SARS-CoV-2 is of utmost importance and a good indicator of exposure and circulation of the virus within the general population. Two serological tools based on a double recognition assay [enzyme-linked immunosorbent assay (DR-ELISA) and lateral flow assay (DR-LFA)] to detect total antibodies to SARS-CoV-2 have been developed based on the recombinant nucleocapsid protein. A total of 1065 serum samples, including positive for COVID-19 and negative samples from healthy donors or infected with other respiratory pathogens, were analyzed. The results showed values of sensitivity between 91.2% and 100%, and specificity of 100% and 98.2% for DR-LFA and DR-ELISA, respectively. No cross-reactivity against seasonal coronavirus (HCoV-NL63, HCoV-229E, HCoV-HKU1, HCoV-OC43) was found. These results demonstrate the importance of serology as a complementary tool to polymerase chain reaction for follow-up of recovered patients and identification of asymptomatic individuals.
Keywords: COVID-19; Diagnosis; ELISA; LFA; SARS-CoV-2; Serology.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Che X.Y., Qiu L.W., Pan Y.X., Wen K., Hao W., Zhang L.Y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004;42:2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

