Current Contributions of Organofluorine Compounds to the Agrochemical Industry
- PMID: 32891056
- PMCID: PMC7479632
- DOI: 10.1016/j.isci.2020.101467
Current Contributions of Organofluorine Compounds to the Agrochemical Industry
Abstract
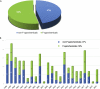
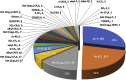
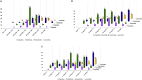
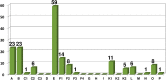
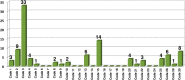
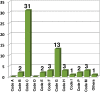
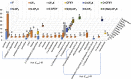
Currently, more than 1,200 agrochemicals are listed and many of these are regularly used by farmers to generate the food supply to support the expanding global population. However, resistance to pesticides is an ever more frequently occurring phenomenon, and thus, a continuous supply of novel agrochemicals with high efficiency, selectivity, and low toxicity is required. Moreover, the demand for a more sustainable society, by reducing the risk chemicals pose to human health and by minimizing their environmental footprint, renders the development of novel agrochemicals an ever more challenging undertaking. In the last two decades, fluoro-chemicals have been associated with significant advances in the agrochemical development process. We herein analyze the contribution that organofluorine compounds make to the agrochemical industry. Our database covers 424 fluoro-agrochemicals and is subdivided into several categories including chemotypes, mode of action, heterocycles, and chirality. This in-depth analysis reveals the unique relationship between fluorine and agrochemicals.
Keywords: Agricultural Science; Chemistry; Industrial Chemistry; Organic Chemistry.
© 2020 The Author(s).
Figures













References
-
- Ames B.N., Gold S.L. Environmental pollution, pesticides, and the prevention of cancer: misconceptions. FASEB J. 1997;11:1041–1052. - PubMed
-
- Arnesen A.K.M. Availability of fluoride to plants grown in contaminated soils. Plant Soil. 1997;191:13–25.
-
- Benskin J.P., Muir D.C.G., Scott B.F., Spencer C., De Silva A.O., Kylin H., Martin J.W., Morris A., Lohmann R., Tomy G. Perfluoroalkyl acids in the atlantic and Canadian Arctic oceans. Environ. Sci. Technol. 2012;46:5815–5823. - PubMed
-
- Besset T., Jubault P., Pannecoucke X., Poisson T. New entries toward the synthesis of OCF3-containing molecules. Org. Chem. Front. 2016;3:1004–1010.
-
- Bhattacharyya A., Barik S.R., Ganguly P. New pesticide molecules, formulation technology and uses: present status and future challenges. J. Plant Protect. Sci. 2009;1:9–15.
Publication types
LinkOut - more resources
Full Text Sources

