HLA-B*35:01 and Green Tea-Induced Liver Injury
- PMID: 32892374
- PMCID: PMC8052949
- DOI: 10.1002/hep.31538
HLA-B*35:01 and Green Tea-Induced Liver Injury
Abstract
Background and aims: Herbal supplements, and particularly multi-ingredient products, have become increasingly common causes of acute liver injury. Green tea is a frequent component in implicated products, but its role in liver injury is controversial. The aim of this study was to better characterize the clinical features, outcomes, and pathogenesis of green tea-associated liver injury.
Approach and results: Among 1,414 patients enrolled in the U.S. Drug-Induced Liver Injury Network who underwent formal causality assessment, 40 cases (3%) were attributed to green tea, 202 to dietary supplements without green tea, and 1,142 to conventional drugs. The clinical features of green tea cases and representation of human leukocyte antigen (HLA) class I and II alleles in cases and control were analyzed in detail. Patients with green tea-associated liver injury ranged in age from 17 to 69 years (median = 40) and developed symptoms 15-448 days (median = 72) after starting the implicated agent. The liver injury was typically hepatocellular (95%) with marked serum aminotransferase elevations and only modest increases in alkaline phosphatase. Most patients were jaundiced (83%) and symptomatic (88%). The course was judged as severe in 14 patients (35%), necessitating liver transplantation in 3 (8%), but rarely resulting in chronic injury (3%). In three instances, injury recurred upon re-exposure to green tea with similar clinical features, but shorter time to onset. HLA typing revealed a high prevalence of HLA-B*35:01, found in 72% (95% confidence interval [CI], 58-87) of green tea cases, but only 15% (95% CI, 10-20) caused by other supplements and 12% (95% CI, 10-14) attributed to drugs, the latter rate being similar to population controls (11%; 95% CI, 10.5-11.5).
Conclusions: Green tea-related liver injury has distinctive clinical features and close association with HLA-B*35:01, suggesting that it is idiosyncratic and immune mediated.
© 2020 by the American Association for the Study of Liver Diseases.
Figures
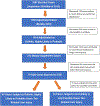
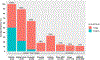
References
-
- Vega M, Verman M, Beswick D, Bey S, Hossack J, Merriman N, et al.; Drug Induced Liver Injury Network (DILIN). The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the State of Delaware. Drug Saf. 2017; 40(9): 783–7. - PMC - PubMed
-
- Cohen PA, Sharfstein J. The opportunity of CBD – reforming the law. N Engl J Med. 2019; 381(4): 297–9. - PubMed
-
- U.S. Food and Drug Administration. Dietary supplements. Available at: http://www.fda.gov/Food/DietarySupplements/default.htm. Accessed January 3, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

