Astrocyte-specific deletion of the transcription factor Yin Yang 1 in murine substantia nigra mitigates manganese-induced dopaminergic neurotoxicity
- PMID: 32893191
- PMCID: PMC7667968
- DOI: 10.1074/jbc.RA120.015552
Astrocyte-specific deletion of the transcription factor Yin Yang 1 in murine substantia nigra mitigates manganese-induced dopaminergic neurotoxicity
Abstract
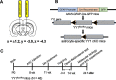
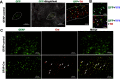
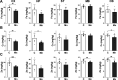
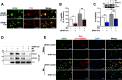
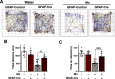
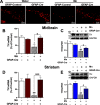
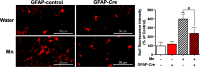
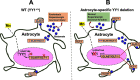
Manganese (Mn)-induced neurotoxicity resembles Parkinson's disease (PD), but the mechanisms underpinning its effects remain unknown. Mn dysregulates astrocytic glutamate transporters, GLT-1 and GLAST, and dopaminergic function, including tyrosine hydroxylase (TH). Our previous in vitro studies have shown that Mn repressed GLAST and GLT-1 via activation of transcription factor Yin Yang 1 (YY1). Here, we investigated if in vivo astrocytic YY1 deletion mitigates Mn-induced dopaminergic neurotoxicity, attenuating Mn-induced reduction in GLAST/GLT-1 expression in murine substantia nigra (SN). AAV5-GFAP-Cre-GFP particles were infused into the SN of 8-week-old YY1 flox/flox mice to generate a region-specific astrocytic YY1 conditional knockout (cKO) mouse model. 3 weeks after adeno-associated viral (AAV) infusion, mice were exposed to 330 μg of Mn (MnCl2 30 mg/kg, intranasal instillation, daily) for 3 weeks. After Mn exposure, motor functions were determined in open-field and rotarod tests, followed by Western blotting, quantitative PCR, and immunohistochemistry to assess YY1, TH, GLAST, and GLT-1 levels. Infusion of AAV5-GFAP-Cre-GFP vectors into the SN resulted in region-specific astrocytic YY1 deletion and attenuation of Mn-induced impairment of motor functions, reduction of TH-expressing cells in SN, and TH mRNA/protein levels in midbrain/striatum. Astrocytic YY1 deletion also attenuated the Mn-induced decrease in GLAST/GLT-1 mRNA/protein levels in midbrain. Moreover, YY1 deletion abrogated its interaction with histone deacetylases in astrocytes. These results indicate that astrocytic YY1 plays a critical role in Mn-induced neurotoxicity in vivo, at least in part, by reducing astrocytic GLAST/GLT-1. Thus, YY1 might be a potential target for treatment of Mn toxicity and other neurological disorders associated with dysregulation of GLAST/GLT-1.
Keywords: GLASTGLT-1; Parkinson disease; adeno-associated viral (AAV); adeno-associated viral vector; animal model; astrocyte; dopaminergic neurotoxicity; glutamate; manganese; manganese Yin Yang 1; tyrosine hydroxylase.
© 2020 Pajarillo et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article..
Figures


References
-
- Keen C. L., Ensunsa J. L., Watson M. H., Baly D. L., Donovan S. M., Monaco M. H., and Clegg M. S. (1999) Nutritional aspects of manganese from experimental studies. Neurotoxicology 20, 213–223 - PubMed
-
- Bowler R. M., Kornblith E. S., Gocheva V. V., Colledge M. A., Bollweg G., Kim Y., Beseler C. L., Wright C. W., Adams S. W., and Lobdell D. T. (2015) Environmental exposure to manganese in air: Associations with cognitive functions. Neurotoxicology 49, 139–148 10.1016/j.neuro.2015.06.004 - DOI - PMC - PubMed
-
- Kullar S. S., Shao K., Surette C., Foucher D., Mergler D., Cormier P., Bellinger D. C., Barbeau B., Sauvé S., and Bouchard M. F. (2019) A benchmark concentration analysis for manganese in drinking water and IQ deficits in children. Environ. Int. 130, 104889 10.1016/j.envint.2019.05.083 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

