Sarcolemma wounding activates dynamin-dependent endocytosis in striated muscle
- PMID: 32893434
- PMCID: PMC9720729
- DOI: 10.1111/febs.15556
Sarcolemma wounding activates dynamin-dependent endocytosis in striated muscle
Abstract
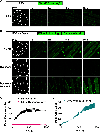
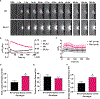
Plasma membrane repair is an evolutionarily conserved mechanism by which cells can seal breaches in the plasma membrane. Mutations in several proteins with putative roles in sarcolemma integrity, membrane repair, and membrane transport result in several forms of muscle disease; however, the mechanisms that are activated and responsible for sarcolemma resealing are not well understood. Using the standard assays for membrane repair, which track the uptake of FM 1-43 dye into adult skeletal muscle fibers following laser-induced sarcolemma disruption, we show that labeling of resting fibers by FM1-43 prior to membrane wounding and the induced FM1-43 dye uptake after sarcolemma wounding occurs via dynamin-dependent endocytosis. Dysferlin-deficient muscle fibers show elevated dye uptake following wounding, which is the basis for the assertion that membrane repair is defective in this model. Our data show that dynamin inhibition mitigates the differences in FM1-43 dye uptake between dysferlin-null and wild-type muscle fibers, suggesting that elevated wound-induced FM1-43 uptake in dysferlin-deficient muscle may actually be due to enhanced dynamin-dependent endocytosis following wounding, though dynamin inhibition had no effect on dysferlin trafficking after wounding. By monitoring calcium flux after membrane wounding, we show that reversal of calcium precedes the sustained, slower increase of dynamin-dependent FM1-43 uptake in WT fibers, and that dysferlin-deficient muscle fibers have persistently increased calcium after wounding, consistent with its proposed role in resealing. These data highlight a previously unappreciated role for dynamin-dependent endocytosis in wounded skeletal muscle fibers and identify overactive dynamin-dependent endocytosis following sarcolemma wounding as a potential mechanism or consequence of dysferlin deficiency.
Keywords: dynamin; dysferlin; endocytosis; membrane repair; membrane transport; skeletal muscle.
© 2020 Federation of European Biochemical Societies.
Conflict of interest statement
Figures


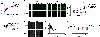


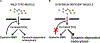
References
-
- Claflin DR & Brooks SV (2008) Direct observation of failing fibers in muscles of dystrophic mice provides mechanistic insight into muscular dystrophy, American Journal of Physiology - Cell Physiology. 294, C651–C658. - PubMed
-
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira E. d. S., Zatz M, Beckmann JS & Bushby K (1998) A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B, Nature Genetics. 20, 37–42. - PubMed
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen C-C, Williamson R, McNeil PL & Campbell KP (2003) Defective membrane repair in dysferlin-deficient muscular dystrophy, Nature. 423, 168–172. - PubMed
-
- Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT & Brown RH (2003) Dysferlin Interacts with Annexins A1 and A2 and Mediates Sarcolemmal Wound-healing, Journal of Biological Chemistry. 278, 50466–50473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

