Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: the role of mucosal strengthening
- PMID: 32893715
- PMCID: PMC7524297
- DOI: 10.1080/19490976.2020.1800897
Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: the role of mucosal strengthening
Abstract
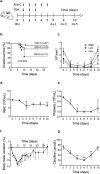
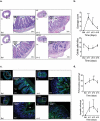
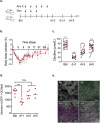
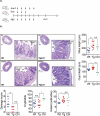
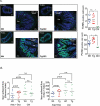
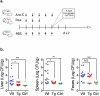
Induction chemotherapy (7 + 3 regimen) remains the gold standard for patients with acute myeloid leukemia (AML) but is responsible for gut damage leading to several complications such as bloodstream infection (BSI). We aimed to investigate the impact of induction chemotherapy on the intestinal barrier of patients with AML and in wild-type mice. Next, we assessed the potential benefit of strengthening the mucosal barrier in transgenic mice releasing a recombinant protein able to reinforce the mucus layer (Tg222). In patients, we observed a decrease of plasma citrulline, which is a marker of the functional enterocyte mass, of short-chain fatty acids and of fecal bacterial load, except for Escherichia coli and Enterococcus spp., which became dominant. Both the α and β-diversities of fecal microbiota decreased. In wild-type mice, citrulline levels decreased under chemotherapy along with an increase of E. coli and Enterococcus spp load associated with concomitant histologic impairment. By comparison with wild-type mice, Tg222 mice, 3 days after completing chemotherapy, had higher citrulline levels, a faster healing epithelium, and preserved α-diversity of their intestinal microbiota. This was associated with reduced bacterial translocations. Our results highlight the intestinal damage and the dysbiosis induced by the 7 + 3 regimen. As a proof of concept, our transgenic model suggests that strengthening the intestinal barrier is a promising approach to limit BSI and improve AML patients' outcome.
Keywords: Intestinal barrier; acute leukemia; chemotherapy; microbiota; mucus.
Figures


References
-
- Lee JH, Kim H, Joo YD, Lee WS, Bae SH, Young Zang D, Kwon J, Kim MK, Lee J, Lee GW, et al. Prospective randomized comparison of idarubicin and high-dose daunorubicin in induction chemotherapy for newly diagnosed acute myeloid leukemia. J Clin Oncol. 2017;35(24):2754–17. doi:10.1200/JCO.2017.72.8618. - DOI - PubMed
-
- Kolonen A, Sinisalo M, Huttunen R, Syrjanën J, Aittoniemi J, Huhtala H, Sankelo M, Rintala H, Räty R, Jantunen E, et al. Bloodstream infections in acute myeloid leukemia patients treated according to the Finnish Leukemia Group AML-2003 protocol – a prospective nationwide study. Infect Dis (Auckl). 2017;49(11–12):799–808. doi:10.1080/23744235.2017.1347814. - DOI - PubMed
-
- Conn JR, Catchpoole EM, Runnegar N, Mapp SJ, Markey KA.. Low rates of antibiotic resistance and infectious mortality in a cohort of high-risk hematology patients: A single center, retrospective analysis of blood stream infection. PLoS One. 2017;12(5):1–13. doi:10.1371/journal.pone.0178059. - DOI - PMC - PubMed
-
- Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MRM, Kamboj M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–4341. doi:10.1172/JCI43918. - DOI - PMC - PubMed
-
- Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevallier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi:10.1186/1741-7007-11-61. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
