Muscular weakness and muscle wasting in the critically ill
- PMID: 32893974
- PMCID: PMC7749542
- DOI: 10.1002/jcsm.12620
Muscular weakness and muscle wasting in the critically ill
Abstract
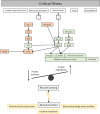
Background: Muscular weakness and/or muscle wasting is recognized as a key medical problem in critically ill patients on intensive care units (ICUs) worldwide.
Methods and results: Intensive care unit-acquired weakness (ICUAW) results from various diseases leading to critical illness and is observed in about 40% [1080/2686 patients, 95% confidence interval (CI): 38-42%] of mixed (medical-surgical) ICU patients. Muscle strength at ICU discharge is directly associated with mortality 5 years after discharge [hazard ratio 0.946, 95% CI: 0.928-0.968 per point increase in Medical Research Council (MRC) scores, P = 0.001]. ICUAW serves as umbrella term for the subgroups ‘critical illness myopathy’, ‘critical illness polyneuropathy’, and ‘critical illness polyneuromyopathy’, the latter distinguished using electrophysiology and/or biopsy studies. Diagnosing, studying, and developing treatments for ICUAW among the critically ill seems challenging due to the acuity and severity of the underlying heterogeneous diseases. Ventilator-induced diaphragmatic dysfunction occurs in up to 80% (n = 32/40) of ICUAW patients after mechanical ventilation and mostly results from distinct muscular pathologies, disuse, underlying critical illness, and/or effects imposed directly by mechanical ventilation. Swallowing disorders/dysphagia likely represent an additional (local) neuromuscular dysfunction/ICUAW sequelae and presents in 10.3% (n = 96/933) of mixed medical-surgical ICU survivors, with 60.4% (n = 58/96) of patients remaining dysphagia positive until hospital discharge. Key independent risk factors for dysphagia following mechanical ventilation are baseline neurological disease [odds ratio (OR) 4.45, 95% CI: 2.74-7.24, P < 0.01], emergency admission (OR 2.04, 95% CI: 1.15-3.59, P < 0.01), days on mechanical ventilation (OR 1.19, 95% CI: 1.06-1.34, P < 0.01), days on renal replacement therapy (OR 1.1, 95% CI: 1-1.23, P = 0.03), and disease severity (Acute Physiology and Chronic Health Evaluation II score within first 24 h; OR 1.03, 95% CI: 0.99-1.07, P < 0.01). Dysphagia positivity independently predicts 28-day and 90-day mortality (90-day univariate hazard ratio: 3.74; 95% CI, 2.01-6.95; P < 0.001) and is associated with a 9.2% excess (all-cause) mortality rate.
Conclusions: Neuromuscular weakness and muscle wasting is observed in many survivors of critical illness. ICUAW, ventilator-induced diaphragmatic dysfunction, and dysphagia are associated with complicated and prolonged ICU stay, impaired weaning from mechanical ventilation, impeded rehabilitative measures, and a considerable impact on morbidity and mortality is noted. Future research strategies should further explore underlying pathomechanisms and lead to development of causal treatment strategies.
Keywords: Critical illness myopathy; Critical illness polyneuropathy; Dysphagia; ICU-acquired weakness; Sepsis; Swallowing disorder.
Conflict of interest statement
J.C.S. reports grants from Orion Pharma, Abbott Nutrition International, B. Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, Phagenesis, and Nycomed outside the submitted work. The money went into departmental funds. No personal financial gain applied. All other authors declare no conflict of interest.
Figures

References
-
- Kramer CL. Intensive care unit‐acquired weakness. Neurol Clin 2017;35:723–736. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

