Ensuring protocol compliance and data transparency in clinical trials using Blockchain smart contracts
- PMID: 32894068
- PMCID: PMC7487835
- DOI: 10.1186/s12874-020-01109-5
Ensuring protocol compliance and data transparency in clinical trials using Blockchain smart contracts
Abstract
Background: Clinical Trials (CTs) help in testing and validating the safety and efficacy of newly discovered drugs on specific patient population cohorts. However, these trials usually experience many challenges, such as extensive time frames, high financial cost, regulatory and administrative barriers, and insufficient workforce. In addition, CTs face several data management challenges pertaining to protocol compliance, patient enrollment, transparency, traceability, data integrity, and selective reporting. Blockchain can potentially address such challenges because of its intrinsic features and properties. Although existing literature broadly discusses the applicability of blockchain-based solutions for CTs, only a few studies present their working proof-of-concept.
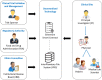
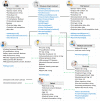
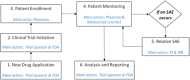
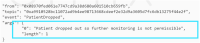
Methods: We propose a blockchain-based framework for CT data management, using Ethereum smart contracts, which employs IPFS as the file storage system to automate processes and information exchange among CT stakeholders. CT documents stored in the IPFS are difficult to tamper with as they are given unique cryptographic hashes. We present algorithms that capture various stages of CT data management. We develop the Ethereum smart contract using Remix IDE that is validated under different scenarios.
Results: The proposed framework results are advantageous to all stakeholders ensuring transparency, data integrity, and protocol compliance. Although the proposed solution is tested on the Ethereum blockchain platform, it can be deployed in private blockchain networks using their native smart contract technologies. We make our smart contract code publicly available on Github.
Conclusions: We conclude that the proposed framework can be highly effective in ensuring that the trial abides by the protocol and the functions are executed only by the stakeholders who are given permission. It also assures data integrity and promotes transparency and traceability of information among stakeholders.
Keywords: Blockchain; Clinical trials; Ethereum; Healthcare; IPFS; Smart contracts.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures











References
-
- S. Nakamoto, "Bitcoin: A Peer-to-Peer Electronic Cash System," [Online]. Available: https://bitcoin.org/bitcoin.pdf. [Accessed 4 Nov. 2018].
-
- Underwood S. Blockchain beyond technology. Commun ACM. 2016;59(11):15–17. doi: 10.1145/2994581. - DOI
-
- Suliman A, Husain Z, Abououf M, Alblooshi M, Salah K. Monetization of IoT data using smart contracts. IET Networks. 2019;8(1):32–37. doi: 10.1049/iet-net.2018.5026. - DOI
-
- Salah K, Nizamuddin N, Al-Fuqaha A. Blockchain for AI: review and open research challenges. IEEE Access. 2019;7:10127–10149. doi: 10.1109/ACCESS.2018.2890507. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

