Evaluation of the birth plan implementation: a parallel convergent mixed study
- PMID: 32894145
- PMCID: PMC7487561
- DOI: 10.1186/s12978-020-00989-6
Evaluation of the birth plan implementation: a parallel convergent mixed study
Abstract
Background: Pregnancy, birth, and motherhood are among the most important events of every woman's life. Training and participation of mothers in the decision-making process of delivery play an essential role in physical as well as psychosocial preparation of the mother. The healthcare system can improve and enhance the level of care by involving the patient in their self-care process. The aim of the present study is to assess the implementation of the birth plan for the first time in Iran in Tabriz city.
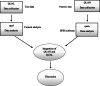
Methods/design: The present study uses a mixed-method with a parallel convergence approach, including both quantitative and qualitative phases. The quantitative phase is a randomized controlled clinical trial performed on 106 pregnant women, 32-36 weeks of pregnancy, referring to Taleghani educational hospital in Tabriz city. The participants will be assigned into intervention and control groups using a randomized block method. A training session will be held about the items of the birth plan checklist at weeks 32-36 of gestation for the participants in the intervention group, whereby a mother-requested birth plan will be developed. It will then be implemented by the researcher after admitting them to the delivery ward. Also, those in the control group will receive routine care. During and after the delivery, the questionnaire of delivery information, neonatal information, and Delivery Fear Scale (DFS) will be completed. Also, a partogram will be completed for all participants by the researcher. The participants in both groups will be followed up until six weeks post-delivery, whereby the instruments of Childbirth Experience Questionnaire (CEQ2.0), Edinburgh's Postpartum Depression Scale and PTSD Symptom Scale 1 (PSS-I) will be completed six weeks 4-6 weeks postpartum by the researcher through an interview with participants in Taleghani educational hospital. The general linear model and multivariate logistic regression model will be used while controlling the possible confounding variables. The qualitative phase will be performed to explore the women's perception of the effect of the birth plan on childbirth experience within 4-6 weeks postpartum. The sampling will be of a purposeful type on the women who would receive the birth plan and will continue until data saturation. In-depth, semi-structured individual interviews would be used for data collection. The data analysis will be done through content analysis with a conventional approach. The results of the quantitative and qualitative phases will be analyzed separately, and then combined in the interpretation stage.
Discussion: By investigating the effect of implementing the birth plan on the childbirth experience of women as well as other maternal and neonatal outcomes, an evidence-based insight can be offered using a culturally sensitive approach. The presentation of the results obtained from this study using the mixed method may be effective in improving the quality of care provided for women during labor.
Trial registration: Iranian Registry of Clinical Trials (IRCT): IRCT20120718010324N58. Date of registration: July 7, 2020. URL: https://en.irct.ir/user/trial/47007/view.
Keywords: Birth experience; Birth plan; Mixed-method.
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Effect of implementing a birth plan on maternal and neonatal outcomes: a randomized controlled trial.BMC Pregnancy Childbirth. 2022 Nov 22;22(1):862. doi: 10.1186/s12884-022-05199-5. BMC Pregnancy Childbirth. 2022. PMID: 36419027 Free PMC article. Clinical Trial.
-
Adaptation and implementation of clinical guidelines on maternal and newborn postnatal care in Iran: study protocol.Reprod Health. 2023 Sep 12;20(1):135. doi: 10.1186/s12978-023-01682-0. Reprod Health. 2023. PMID: 37700318 Free PMC article.
-
Implementation and evaluation of the centering pregnancy group prenatal care model in pregnant women with diabetes: a convergent parallel mixed methods study protocol.Reprod Health. 2024 Apr 18;21(1):54. doi: 10.1186/s12978-024-01792-3. Reprod Health. 2024. PMID: 38637855 Free PMC article.
-
Recommendations for improving primiparous women's childbirth experience: results from a multiphase study in Iran.Reprod Health. 2021 Jul 6;18(1):146. doi: 10.1186/s12978-021-01196-7. Reprod Health. 2021. PMID: 34229710 Free PMC article. Review.
-
Repercussions of using the birth plan in the parturition process.Rev Gaucha Enferm. 2019 Jun 6;40:e20180233. doi: 10.1590/1983-1447.2019.20180233. Rev Gaucha Enferm. 2019. PMID: 31188973 English, Portuguese.
Cited by
-
Desired versus actual delivery route: nursing students' perception about their type of delivery.Rev Esc Enferm USP. 2022 Dec 5;56:e20220217. doi: 10.1590/1980-220X-REEUSP-2022-0217en. eCollection 2022. Rev Esc Enferm USP. 2022. PMID: 36477249 Free PMC article.
-
The childbirth experiences of Iranian women with birth plans.Heliyon. 2024 Sep 6;10(17):e37555. doi: 10.1016/j.heliyon.2024.e37555. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39290261 Free PMC article.
-
Maternal-infant outcomes of birth planning: A review study.J Educ Health Promot. 2023 Sep 29;12:315. doi: 10.4103/jehp.jehp_1450_22. eCollection 2023. J Educ Health Promot. 2023. PMID: 38023070 Free PMC article. Review.
-
Effect of implementing a birth plan on maternal and neonatal outcomes: a randomized controlled trial.BMC Pregnancy Childbirth. 2022 Nov 22;22(1):862. doi: 10.1186/s12884-022-05199-5. BMC Pregnancy Childbirth. 2022. PMID: 36419027 Free PMC article. Clinical Trial.
References
-
- Hildingsson I, Johansson M, Karlström A, Fenwick J. Factors associated with a positive birth experience: an exploration of swedish women's experiences. Int J Childbirth. 2013;3(3):153–164. doi: 10.1891/2156-5287.3.3.153. - DOI
-
- Hollins Martin CJ. Birth planning for midwives and mothers. Br J Midwifery. 2008;16(9):583–587. doi: 10.12968/bjom.2008.16.9.30881. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials