Histone chaperone FACT represses retrotransposon MERVL and MERVL-derived cryptic promoters
- PMID: 32894293
- PMCID: PMC7544220
- DOI: 10.1093/nar/gkaa732
Histone chaperone FACT represses retrotransposon MERVL and MERVL-derived cryptic promoters
Abstract
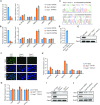
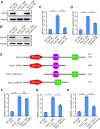
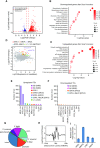
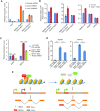
Endogenous retroviruses (ERVs) were usually silenced by various histone modifications on histone H3 variants and respective histone chaperones in embryonic stem cells (ESCs). However, it is still unknown whether chaperones of other histones could repress ERVs. Here, we show that H2A/H2B histone chaperone FACT plays a critical role in silencing ERVs and ERV-derived cryptic promoters in ESCs. Loss of FACT component Ssrp1 activated MERVL whereas the re-introduction of Ssrp1 rescued the phenotype. Additionally, Ssrp1 interacted with MERVL and suppressed cryptic transcription of MERVL-fused genes. Remarkably, Ssrp1 interacted with and recruited H2B deubiquitinase Usp7 to Ssrp1 target genes. Suppression of Usp7 caused similar phenotypes as loss of Ssrp1. Furthermore, Usp7 acted by deubiquitinating H2Bub and thereby repressed the expression of MERVL-fused genes. Taken together, our study uncovers a unique mechanism by which FACT complex silences ERVs and ERV-derived cryptic promoters in ESCs.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Nsd2 Represses Endogenous Retrovirus MERVL in Embryonic Stem Cells.Stem Cells Int. 2021 Jan 16;2021:6663960. doi: 10.1155/2021/6663960. eCollection 2021. Stem Cells Int. 2021. PMID: 33531910 Free PMC article.
-
Dot1l cooperates with Npm1 to repress endogenous retrovirus MERVL in embryonic stem cells.Nucleic Acids Res. 2023 Sep 22;51(17):8970-8986. doi: 10.1093/nar/gkad640. Nucleic Acids Res. 2023. PMID: 37522386 Free PMC article.
-
Regulation of chromatin structure and function: insights into the histone chaperone FACT.Cell Cycle. 2021 Mar-Mar;20(5-6):465-479. doi: 10.1080/15384101.2021.1881726. Epub 2021 Feb 16. Cell Cycle. 2021. PMID: 33590780 Free PMC article. Review.
-
Zscan4c activates endogenous retrovirus MERVL and cleavage embryo genes.Nucleic Acids Res. 2019 Sep 19;47(16):8485-8501. doi: 10.1093/nar/gkz594. Nucleic Acids Res. 2019. PMID: 31304534 Free PMC article.
-
Mechanistic and structural insights into histone H2A-H2B chaperone in chromatin regulation.Biochem J. 2020 Sep 18;477(17):3367-3386. doi: 10.1042/BCJ20190852. Biochem J. 2020. PMID: 32941645 Review.
Cited by
-
The regulation of totipotency transcription: Perspective from in vitro and in vivo totipotency.Front Cell Dev Biol. 2022 Oct 26;10:1024093. doi: 10.3389/fcell.2022.1024093. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393839 Free PMC article. Review.
-
Elevated RIF1 participates in the epigenetic abnormalities of zygotes by regulating histone modifications on MuERV-L in obese mice.Mol Med. 2022 Feb 5;28(1):17. doi: 10.1186/s10020-022-00446-z. Mol Med. 2022. PMID: 35123389 Free PMC article.
-
Rap1 regulates TIP60 function during fate transition between two-cell-like and pluripotent states.Genes Dev. 2022 Mar 1;36(5-6):313-330. doi: 10.1101/gad.349039.121. Epub 2022 Feb 24. Genes Dev. 2022. PMID: 35210222 Free PMC article.
-
FACT subunit SUPT16H associates with BRD4 and contributes to silencing of interferon signaling.Nucleic Acids Res. 2022 Aug 26;50(15):8700-8718. doi: 10.1093/nar/gkac645. Nucleic Acids Res. 2022. PMID: 35904816 Free PMC article.
-
Exploiting the DNA Damage Response for Prostate Cancer Therapy.Cancers (Basel). 2023 Dec 23;16(1):83. doi: 10.3390/cancers16010083. Cancers (Basel). 2023. PMID: 38201511 Free PMC article. Review.
References
-
- Goke J., Lu X., Chan Y.S., Ng H.H., Ly L.H., Sachs F., Szczerbinska I.. Dynamic transcription of distinct classes of endogenous retroviral elements marks specific populations of early human embryonic cells. Cell Stem Cell. 2015; 16:135–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

