Pathogenesis of preterm birth: bidirectional inflammation in mother and fetus
- PMID: 32894326
- PMCID: PMC7508962
- DOI: 10.1007/s00281-020-00807-y
Pathogenesis of preterm birth: bidirectional inflammation in mother and fetus
Abstract
Preterm birth (PTB) complicates 5-18% of pregnancies globally and is a leading cause of maternal and fetal morbidity and mortality. Most PTB is spontaneous and idiopathic, with largely undefined causes. To increase understanding of PTB, much research in recent years has focused on using animal models to recapitulate the pathophysiology of PTB. Dysfunctions of maternal immune adaptations have been implicated in a range of pregnancy pathologies, including PTB. A wealth of evidence arising from mouse models as well as human studies is now available to support that PTB results from a breakdown in fetal-maternal tolerance, along with excessive, premature inflammation. In this review, we examine the current knowledge of the bidirectional communication between fetal and maternal systems and its role in the immunopathogenesis of PTB. These recent insights significantly advance our understanding of the pathogenesis of PTB, which is essential to ultimately designing more effective strategies for early prediction and subsequent prevention of PTB.
Keywords: Fetal signals; Inflammatory signaling pathways; Labor; Microbiome; Mouse models; Preterm birth; Regulatory T cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


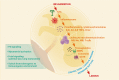
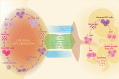
References
-
- Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, Basterrechea M, Bisgaard H, Chatzi L, Corpeleijn E, Correia S, Craig LC, Devereux G, Dogaru C, Dostal M, Duchen K, Eggesbo M, van der Ent CK, Fantini MP, Forastiere F, Frey U, Gehring U, Gori D, van der Gugten AC, Hanke W, Henderson AJ, Heude B, Iniguez C, Inskip HM, Keil T, Kelleher CC, Kogevinas M, Kreiner-Moller E, Kuehni CE, Kupers LK, Lancz K, Larsen PS, Lau S, Ludvigsson J, Mommers M, Nybo Andersen AM, Palkovicova L, Pike KC, Pizzi C, Polanska K, Porta D, Richiardi L, Roberts G, Schmidt A, Sram RJ, Sunyer J, Thijs C, Torrent M, Viljoen K, Wijga AH, Vrijheid M, Jaddoe VW, Duijts L. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133(5):1317–1329. doi: 10.1016/j.jaci.2013.12.1082. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

