[The modified treat and extend scheme with injection blocks in intravitreal injection treatment : Retrospective analysis from the routine clinical application]
- PMID: 32894329
- PMCID: PMC8187201
- DOI: 10.1007/s00347-020-01218-y
[The modified treat and extend scheme with injection blocks in intravitreal injection treatment : Retrospective analysis from the routine clinical application]
Abstract
Background: Regular treatment with intravitreal operative medication injections (IVOM) and the associated frequent check-ups are a major challenge for many patients, which can lead to treatment discontinuation. The aim of the modified treat and extend (TAE) in blocks scheme is to achieve stable retinal and visual outcomes with as few control visits as possible and thus to minimize the burden for the individual patient.
Methods: This monocentric retrospective study examined the treatment courses of 387 patients with neovascular age-related macular degeneration, diabetic macular edema and retinal vein occlusion, who were treated by three blocks of intravitreal injections in the TAE regimen. The primary endpoint was achieving an injection interval of 12 weeks.
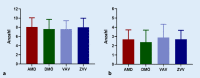
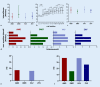
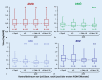
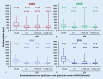
Results: Intravitreal injections in TAE blocks significantly reduced retinal thickness and improved visual acuity. On average, a treatment interval of 2 months was achieved across the various indications.
Conclusion: Intravitreal injections in TAE blocks of three injections can reduce patients' burden and lead to stable retinal and visual results.
Hintergrund: Die regelmäßige Therapie mittels intravitrealer operativer Medikamentenapplikation (IVOM) und die damit verbundenen Kontrolluntersuchungen stellen für viele Patienten eine große Herausforderung dar, die bis zum Behandlungsabbruch führen kann. Das modifizierte blockweise Treat-and-Extend(TAE)-Schema verfolgt das Ziel, mit möglichst wenigen Kontrollvisiten stabile Netzhaut- und Visusbefunde zu erreichen und dadurch die Belastung der Patienten zu minimieren.
Methoden: Diese monozentrische retrospektive Studie untersuchte Behandlungsverläufe von insgesamt 387 Patienten mit neovaskulärer altersbedingter Makuladegeneration (AMD), diabetischem Makulaödem (DMÖ), Venenastverschluss (VAV) und Zentralvenenverschluss (ZVV), bei denen das TAE-Regime jeweils in 3er-Blöcken angewendet wurde. Primärer Endpunkt war das Erreichen eines Injektionsintervalls von 12 Wochen.
Ergebnisse: Durch die blockweise applizierte IVOM konnte die Netzhautdicke signifikant reduziert und der Visus verbessert werden. Über die verschiedenen Indikationen konnte im Mittel ein Behandlungsintervall von 2 Monaten erreicht werden.
Schlussfolgerung: Ein in 3er-Blöcken angepasstes TAE-Schema im Rahmen der IVOM kann bei reduzierter Patientenbelastung zu stabilen Netzhaut- und Visusbefunden führen.
Keywords: Age-related macular degeneration; Anti-VEGF; Diabetic macular edema; Retinal vein occlusion; Treat and extend.
References
-
- Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, Adamis AP, Rubio RG, Murahashi WY. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–1602. doi: 10.1016/j.ophtha.2011.02.022. - DOI - PubMed
-
- Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–2022. doi: 10.1016/j.ophtha.2013.02.034. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical