Epoxy Fatty Acids Are Promising Targets for Treatment of Pain, Cardiovascular Disease and Other Indications Characterized by Mitochondrial Dysfunction, Endoplasmic Stress and Inflammation
- PMID: 32894508
- PMCID: PMC7737916
- DOI: 10.1007/978-3-030-50621-6_5
Epoxy Fatty Acids Are Promising Targets for Treatment of Pain, Cardiovascular Disease and Other Indications Characterized by Mitochondrial Dysfunction, Endoplasmic Stress and Inflammation
Abstract
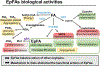
Bioactive lipid mediators resulting from the metabolism of polyunsaturated fatty acids (PUFA) are controlled by many pathways that regulate the levels of these mediators and maintain homeostasis to prevent disease. PUFA metabolism is driven primarily through three pathways. Two pathways, the cyclooxygenase (COX) and lipoxygenase (LO) enzymatic pathways, form metabolites that are mostly inflammatory, while the third route of metabolism results from the oxidation by the cytochrome P450 enzymes to form hydroxylated PUFA and epoxide metabolites. These epoxygenated fatty acids (EpFA) demonstrate largely anti-inflammatory and beneficial properties, in contrast to the other metabolites formed from the degradation of PUFA. Dysregulation of these systems often leads to chronic disease. Pharmaceutical targets of disease focus on preventing the formation of inflammatory metabolites from the COX and LO pathways, while maintaining the EpFA and increasing their concentration in the body is seen as beneficial to treating and preventing disease. The soluble epoxide hydrolase (sEH) is the major route of metabolism of EpFA. Inhibiting its activity increases concentrations of beneficial EpFA, and often disease states correlate to mutations in the sEH enzyme that increase its activity and decrease the concentrations of EpFA in the body. Recent approaches to increasing EpFA include synthetic mimics that replicate biological activity of EpFA while preventing their metabolism, while other approaches focus on developing small molecule inhibitors to the sEH. Increasing EpFA concentrations in the body has demonstrated multiple beneficial effects in treating many diseases, including inflammatory and painful conditions, cardiovascular disease, neurological and disease of the central nervous system. Demonstration of efficacy in so many disease states can be explained by the fundamental mechanism that EpFA have of maintaining healthy microvasculature and preventing mitochondrial and endoplasmic reticulum stress. While there are no FDA approved methods that target the sEH or other enzymes responsible for metabolizing EpFA, current clinical efforts to test for efficacy by increasing EpFA that include inhibiting the sEH or administration of EpFA mimics that block metabolism are in progress.
Keywords: Epoxide; Lipid metabolism; Oxylipin; PUFA; sEH.
Conflict of interest statement
Figures
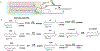
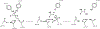

References
-
- J H. Essential fatty acids. Linus Pauling Institute, 2003. [cited 2019]. Available from: https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

