Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection
- PMID: 32895065
- PMCID: PMC7520631
- DOI: 10.1017/ice.2020.454
Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection
Erratum in
-
Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection - ERRATUM.Infect Control Hosp Epidemiol. 2021 Feb;42(2):250. doi: 10.1017/ice.2020.1380. Epub 2020 Dec 17. Infect Control Hosp Epidemiol. 2021. PMID: 33327986 Free PMC article. No abstract available.
Abstract
Objectives: The coronavirus disease 2019 (COVID-19) pandemic has induced a reinforcement of infection control measures in the hospital setting. Here, we assess the impact of the COVID-19 pandemic on the incidence of nosocomial Clostridioides difficile infection (CDI).
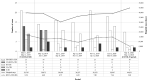
Methods: We retrospectively compared the incidence density (cases per 10,000 patient days) of healthcare-facility-associated (HCFA) CDI in a tertiary-care hospital in Madrid, Spain, during the maximum incidence of COVID-19 (March 11 to May 11, 2020) with the same period of the previous year (control period). We also assessed the aggregate in-hospital antibiotic use (ie, defined daily doses [DDD] per 100 occupied bed days [BD]) and incidence density (ie, movements per 1,000 patient days) of patient mobility during both periods.
Results: In total, 2,337 patients with reverse transcription-polymerase chain reaction-confirmed COVID-19 were admitted to the hospital during the COVID-19 period. Also, 12 HCFA CDI cases were reported at this time (incidence density, 2.68 per 10,000 patient days), whereas 34 HCFA CDI cases were identified during the control period (incidence density, 8.54 per 10,000 patient days) (P = .000257). Antibiotic consumption was slightly higher during the COVID-19 period (89.73 DDD per 100 BD) than during the control period (79.16 DDD per 100 BD). The incidence density of patient movements was 587.61 per 1,000 patient days during the control period and was significantly lower during the COVID-19 period (300.86 per 1,000 patient days) (P < .0001).
Conclusions: The observed reduction of ~70% in the incidence density of HCFA CDI in a context of no reduction in antibiotic use supports the importance of reducing nosocomial transmission by healthcare workers and asymptomatic colonized patients, reinforcing cleaning procedures and reducing patient mobility in the epidemiological control of CDI.
Conflict of interest statement
All authors report no conflicts of interest relevant to this article.
Figures
References
-
- Davies KA, Longshaw CM, Davis GL, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis 2014;14:1208–1219. - PubMed
-
- Longo DL, Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015;372:1539–1548. - PubMed
-
- Tschudin-Sutter S, Kuijper EJ, Durovic A, et al. Guidance document for prevention of Clostridium difficile infection in acute healthcare settings. Clin Microbiol Infect 2018;24:1051–1054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

