Prevalence of TB symptoms, diagnosis and treatment among people living with HIV (PLHIV) not on ART presenting at outpatient clinics in South Africa and Kenya: baseline results from a clinical trial
- PMID: 32895266
- PMCID: PMC7476481
- DOI: 10.1136/bmjopen-2019-035794
Prevalence of TB symptoms, diagnosis and treatment among people living with HIV (PLHIV) not on ART presenting at outpatient clinics in South Africa and Kenya: baseline results from a clinical trial
Abstract
Objective: We used screening data and routine clinic records for intervention arm patients in the Simplified Algorithm for Treatment Eligibility (SLATE) trials to describe the prevalence of tuberculosis (TB) symptoms, diagnosis and treatment among people living with HIV (PLHIV), not on antiretroviral therapy (ART) and presenting at outpatient clinics in South Africa and Kenya. We compared the performance of the WHO four-symptom TB screening tool with a baseline Xpert test.
Setting: Outpatient HIV clinics in South Africa and Kenya.
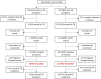
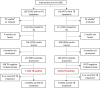
Participants: Eligible patients were non-pregnant, PLHIV, >18 years of age, not on ART, willing to provide written informed consent. A total of 594 patients in South Africa and 240 in Kenya were eligible.
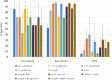
Results: Prevalence of any TB symptom was 38% in Kenya, 35% (SLATE I) and 47% (SLATE II) in South Africa. During SLATE I, 70% of patients in Kenya and 57% in South Africa with ≥1 TB symptom were tested for TB. In SLATE II, 79% of patients with ≥1 TB symptom were tested. Of those, 19% tested positive for TB in Kenya, 15% (SLATE I) and 5% (SLATE II) tested positive in South Africa. Of the 28 patients who tested positive in both trials, 20 initiated TB treatment. The lowest median CD4 counts were among those with active TB (Kenya 124 cells/mm3; South Africa 193 cells/mm3). When comparing the WHO four-symptom screening tool to the Xpert test (SLATE II), we found that increasing the number of symptoms required for a positive screen from one to three or four decreased sensitivity but increased the positive predictive value to >30%.
Conclusions: 80% of patients assessed for ART initiation presented with ≥1 TB symptoms. Reconsideration of the 'any symptom' rule may be appropriate, with ART initiation among patients with fewer/milder symptoms commencing while TB test results are pending.
Trial registration number: NCT02891135 and NCT03315013.
Keywords: HIV & AIDS; clinical trials; tuberculosis.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- World Health Organization Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: World Health Organization, 2017. - PubMed
-
- National Department of Health Same-day antiretroviral therapy (ART) initiation for HIV positive patients. Pretoria: National Department of Health, 2017.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
