Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): outcomes by prior treatment at first relapse
- PMID: 32895455
- PMCID: PMC8179841
- DOI: 10.1038/s41375-020-01021-3
Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): outcomes by prior treatment at first relapse
Abstract
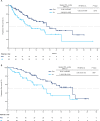
In the phase 3 OPTIMISMM trial, pomalidomide, bortezomib, and dexamethasone (PVd) demonstrated superior efficacy vs bortezomib and dexamethasone (Vd) in patients with relapsed or refractory multiple myeloma previously treated with lenalidomide, including those refractory to lenalidomide. This analysis evaluated outcomes in patients at first relapse (N = 226) by lenalidomide-refractory status, prior bortezomib exposure, and prior stem cell transplant (SCT). Second-line PVd significantly improved PFS vs Vd in lenalidomide-refractory (17.8 vs 9.5 months; P = 0.0276) and lenalidomide-nonrefractory patients (22.0 vs 12.0 months; P = 0.0491), patients with prior bortezomib (17.8 vs 12.0 months; P = 0.0068), and patients with (22.0 vs 13.8 months; P = 0.0241) or without (16.5 vs 9.5 months; P = 0.0454) prior SCT. In patients without prior bortezomib, median PFS was 20.7 vs 9.5 months (P = 0.1055). Significant improvement in overall response rate was also observed with PVd vs Vd in lenalidomide-refractory (85.9% vs 50.8%; P < 0.001) and lenalidomide-nonrefractory (95.7% vs 60.0%; P < 0.001) patients, with similar results regardless of prior bortezomib or SCT. No new safety signals were observed. These data demonstrate the benefit of PVd at first relapse, including immediately after upfront lenalidomide treatment failure and other common first-line treatments.
Conflict of interest statement
MD reports honoraria from Janssen, Celgene, a Bristol Myers Squibb Company, Takeda, Amgen, and Bristol Myers Squibb; KW reports honoraria from Amgen, Bristol Myers Squibb, Celgene, a Bristol Myers Squibb Company, Janssen, and Takeda; consultancy for or serving on board of directors or advisory committee for Amgen, Bristol Myers Squibb, Celgene, a Bristol Myers Squibb Company, Janssen, Juno, Sanofi, and Takeda; PM reports honoraria from serving on boards of directors or advisory committees for Amgen, Celgene, a Bristol Myers Squibb Company, Janssen, Takeda, and AbbVie; LDAJ reports speakers bureau for Celgene, a Bristol Myers Squibb Company, Amgen, and Takeda; DW reports honoraria from serving on boards of directors or advisory committees for Amgen, Celgene, Janssen, and Takeda; JS-M reports honoraria from Janssen, Celgene, a Bristol Myers Squibb Company, Amgen, Bristol Myers Squibb, Novartis, Sanofi, and Roche; PS reports honoraria and research funding from Amgen, Celgene, a Bristol Myers Squibb Company, Janssen, Karyopharm, and Bristol Myers Squibb; ME and AC report nothing to disclose; MJ reports consultancy for and honoraria from serving on boards of directors, advisory committees, and speakers bureaus for Novartis, Janssen, Takeda, Amgen, and Celgene, a Bristol Myers Squibb Company, travel support and research funding from Janssen and Amgen, serving on board of directors or advisory committee for Chugai, and research funding from Takeda and Celgene, a Bristol Myers Squibb Company; JD reports honoraria from Janssen, Celgene, a Bristol Myers Squibb Company, and Roche, consultancy for Janssen, and speakers bureau for Roche; MP reports consultancy for and honoraria from serving on board of directors or advisory committee, and research funding from Celgene, a Bristol Myers Squibb Company, Novartis, AstraZeneca, Bristol Myers Squibb, Roche, Takeda, and Janssen; MS reports consultancy for Janssen; EC, SS, XY, TB, and TP report employment with and equity ownership in Bristol Myers Squibb; TN reports employment with Bristol Myers Squibb; PR reports serving on board of directors or advisory committee for Karyopharm, Oncopeptides, Celgene, a Bristol Myers Squibb Company, Takeda, Amgen, and Jazz Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

