Effects of testosterone replacement on serotonin levels in the prostate and plasma in a murine model of hypogonadism
- PMID: 32895458
- PMCID: PMC7477238
- DOI: 10.1038/s41598-020-71718-z
Effects of testosterone replacement on serotonin levels in the prostate and plasma in a murine model of hypogonadism
Abstract
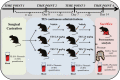
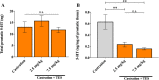
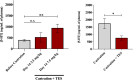
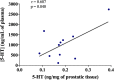
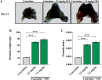
Benign prostate hyperplasia is a dysfunctional disease with an elevated prevalence. Despite the accepted impact of aging and testosterone (TES) in its pathophysiology, its aetiology remains unknown. Recent studies described that serotonin (5-HT) inhibits benign prostate growth through the modulation of the androgen receptor, in the presence of TES. Accordingly, this work aimed to determine the impact of castration and TES replacement in plasmatic and prostatic 5-HT regulation. C57BL/6 mice were submitted to surgical castration and divided into three groups, continually exposed to either vehicle or different TES doses for 14 days. Plasmatic 5-HT concentration was measured before and after castration, and after TES reintroduction. Finally, total prostatic weight and intra-prostatic 5-HT were determined in the different groups. Our results demonstrate that mice prostate exhibits high 5-HT tissue levels and that intra-prostatic total 5-HT was independent of castration or TES reintroduction, in all studied groups. Also, 5-HT plasmatic concentration significantly increased after castration and then normalized after TES administration. Our findings revealed that mice prostate has a high 5-HT content and that total prostatic 5-HT levels do not depend on androgens' action. On the other hand, castration induced a significant increase in plasmatic 5-HT concentration, raising the hypothesis that androgens might be regulating the production of extra-prostatic 5-HT.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

