Prediction of an MMP-1 inhibitor activity cliff using the SAR matrix approach and its experimental validation
- PMID: 32895466
- PMCID: PMC7477548
- DOI: 10.1038/s41598-020-71696-2
Prediction of an MMP-1 inhibitor activity cliff using the SAR matrix approach and its experimental validation
Abstract
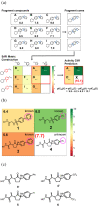
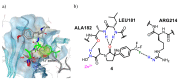
A matrix metalloproteinase 1 (MMP-1) inhibitor activity cliff was predicted using the SAR Matrix method. Compound 4 was predicted as a highly potent activity cliff partner and found to possess 60 times higher inhibitory activity against MMP-1 than the structurally related compound 3. Furthermore, pharmacophore fitting of synthesized compounds indicated that the correctly predicted activity cliff was caused by interactions between the trifluoromethyl group at para position in compound 4 and residue ARG214 of MMP-1.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

