Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure
- PMID: 32895569
- PMCID: PMC7116539
- DOI: 10.1038/s41591-020-1024-z
Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure
Abstract
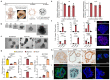
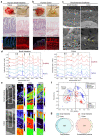
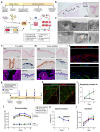
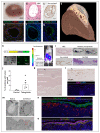
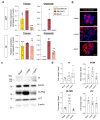
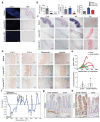
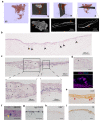
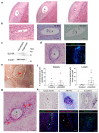
Intestinal failure, following extensive anatomical or functional loss of small intestine, has debilitating long-term consequences for children1. The priority of patient care is to increase the length of functional intestine, particularly the jejunum, to promote nutritional independence2. Here we construct autologous jejunal mucosal grafts using biomaterials from pediatric patients and show that patient-derived organoids can be expanded efficiently in vitro. In parallel, we generate decellularized human intestinal matrix with intact nanotopography, which forms biological scaffolds. Proteomic and Raman spectroscopy analyses reveal highly analogous biochemical profiles of human small intestine and colon scaffolds, indicating that they can be used interchangeably as platforms for intestinal engineering. Indeed, seeding of jejunal organoids onto either type of scaffold reliably reconstructs grafts that exhibit several aspects of physiological jejunal function and that survive to form luminal structures after transplantation into the kidney capsule or subcutaneous pockets of mice for up to 2 weeks. Our findings provide proof-of-concept data for engineering patient-specific jejunal grafts for children with intestinal failure, ultimately aiding in the restoration of nutritional autonomy.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Linking human intestinal scaffolds and organoids to combat intestinal failure.Nat Med. 2020 Oct;26(10):1517-1518. doi: 10.1038/s41591-020-1096-9. Nat Med. 2020. PMID: 32968235 No abstract available.
-
Next-generation intestinal organoids.Nat Rev Gastroenterol Hepatol. 2020 Nov;17(11):649. doi: 10.1038/s41575-020-00371-8. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32994569 No abstract available.
References
-
- O’Keefe SJ, et al. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol. 2006;4:6–10. - PubMed
-
- Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–28. - PubMed
-
- Rama P, et al. Limbal stem-cell therapy and long-term corneal regeneration. The New England journal of medicine. 2010;363:147–155. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

