Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats
- PMID: 32895577
- PMCID: PMC7572652
- DOI: 10.1038/s42255-020-00275-6
Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats
Abstract
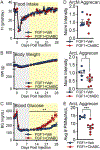
We recently showed that perineuronal nets (PNNs) enmesh glucoregulatory neurons in the arcuate nucleus (Arc) of the mediobasal hypothalamus (MBH)1, but whether these PNNs play a role in either the pathogenesis of type 2 diabetes (T2D) or its treatment remains unclear. Here we show that PNN abundance within the Arc is markedly reduced in the Zucker diabetic fatty (ZDF) rat model of T2D, compared with normoglycaemic rats, correlating with altered PNN-associated sulfation patterns of chondroitin sulfate glycosaminoglycans in the MBH. Each of these PNN-associated changes is reversed following a single intracerebroventricular (icv) injection of fibroblast growth factor 1 (FGF1) at a dose that induces sustained diabetes remission in male ZDF rats. Combined with previous work localizing this FGF1 effect to the Arc area2-4, our finding that enzymatic digestion of Arc PNNs markedly shortens the duration of diabetes remission following icv FGF1 injection in these animals identifies these extracellular matrix structures as previously unrecognized participants in the mechanism underlying diabetes remission induced by the central action of FGF1.
Conflict of interest statement
Figures

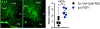



Comment in
-
Hypothalamic Regulation of Glucose Homeostasis: Is the Answer in the Matrix?Cell Metab. 2020 Nov 3;32(5):701-703. doi: 10.1016/j.cmet.2020.10.003. Cell Metab. 2020. PMID: 33147483
References
-
- Blumcke I, Eggli P & Celio MR Relationship between astrocytic processes and “perineuronal nets” in rat neocortex. Glia 15, 131–140 (1995). - PubMed
Methods References
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007028/HL/NHLBI NIH HHS/United States
- R01 GM127579/GM/NIGMS NIH HHS/United States
- R01 DK089056/DK/NIDDK NIH HHS/United States
- F32 DK122662/DK/NIDDK NIH HHS/United States
- R01 DK083042/DK/NIDDK NIH HHS/United States
- T32 AG052354/AG/NIA NIH HHS/United States
- F32 DK108596/DK/NIDDK NIH HHS/United States
- K08 DK114474/DK/NIDDK NIH HHS/United States
- P30 DK035816/DK/NIDDK NIH HHS/United States
- R01 DK101997/DK/NIDDK NIH HHS/United States
- K12 NS080223/NS/NINDS NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

