Managing rheumatic diseases during COVID-19
- PMID: 32895747
- PMCID: PMC7476772
- DOI: 10.1007/s10067-020-05387-8
Managing rheumatic diseases during COVID-19
Abstract
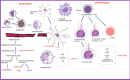
Rheumatology practice, during Coronavirus Disease 2019 (COVID-19) pandemic, has faced multifaceted challenges. Rheumatologists routinely prescribe immunosuppressant medications to their patients with multisystem autoimmune rheumatic diseases who are concerned about the increased risk of acquiring COVID-19 infection and are anxious to know if they should continue or hold these medications. Rheumatologists are often inundated by calls from their patients and physician colleagues caring for COVID-19 patients in hospitals, about how to manage the immunosuppression. Physicians face the challenging task of keeping up with the most up-to-date information on COVID-19. There are uncertainties about the mode of spread, clinical features, management options as well as long-term complications of COVID-19. Data are rapidly evolving and different studies on treatment options are showing contradictory results. It is known that viral illnesses can trigger a flare-up of underlying rheumatic disease that was previously in remission. To further complicate the scenario, some of the immunosuppressants have shown to have antiviral properties. This has created dilemma in the light of current COVID-19 crisis, as whether to continue or stop the immunosuppressive agents which could be essential to prevent complications of the rheumatic diseases including organ failure but also there is concern about acquiring COVID-19 or developing serious infection. Until we get an effective vaccine, immunosuppressant management for rheumatic diseases as well as other autoimmune diseases and transplants will pose difficult questions. This article is an attempt to review and understand COVID-19 and its impact on the immune system with special emphasis on managing medications used for autoimmune rheumatic diseases. We have provided general guidance about decision making, in regards to the immunosuppressive agents used in rheumatology practice with an understanding that this may change in near future.
Keywords: Autoimmune; COVID-19; DMARD; Immunosuppressive; Medications; Rheumatic.
Conflict of interest statement
Amit P. Ladani—none
Muruga Loganathan—none
Abhijeet Danve—Advisory boards and honoraria: Janssen; Research Grants: Novartis
Figures

References
-
- COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. https://www.who.int/publications/m/item/covid-19-public-health-emergency.... Accessed 5 Jul 2020
-
- WHO COVID-19 situation report - 167. 20200705-covid-19-sitrep-167.pdf
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

